Abstract
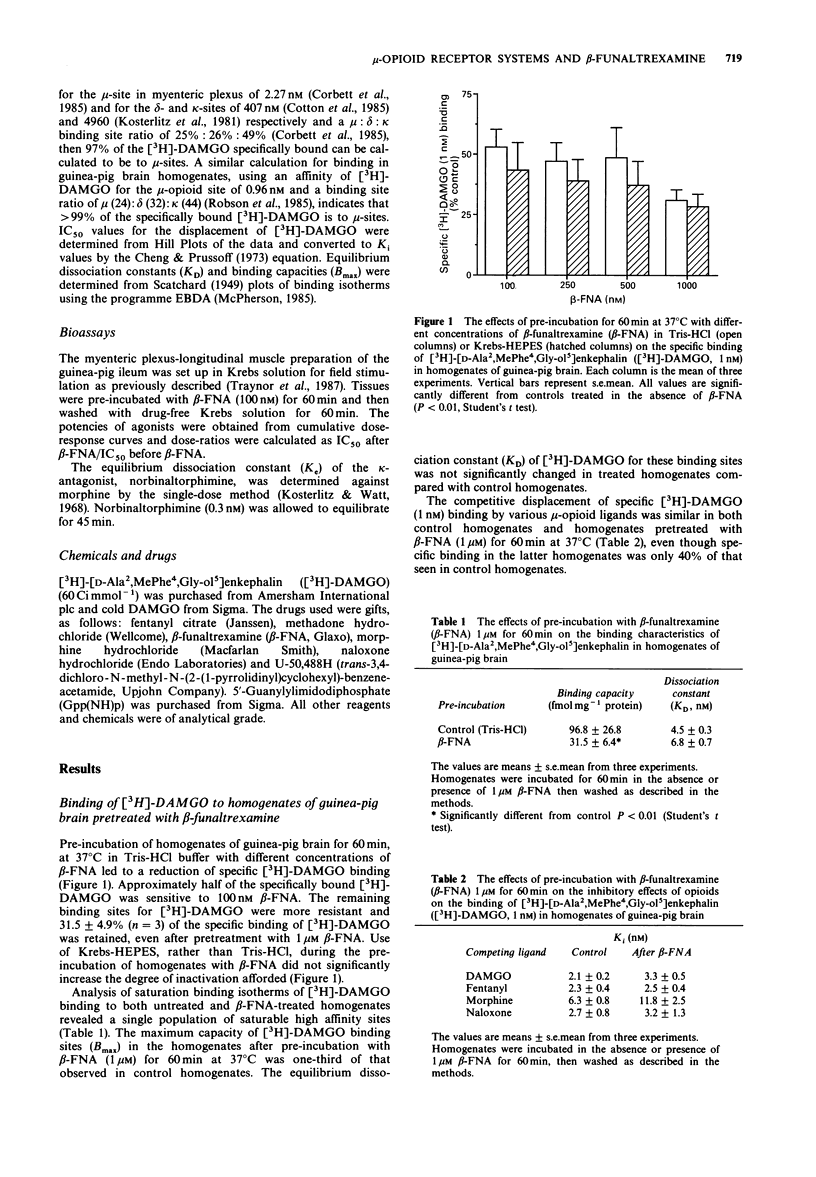
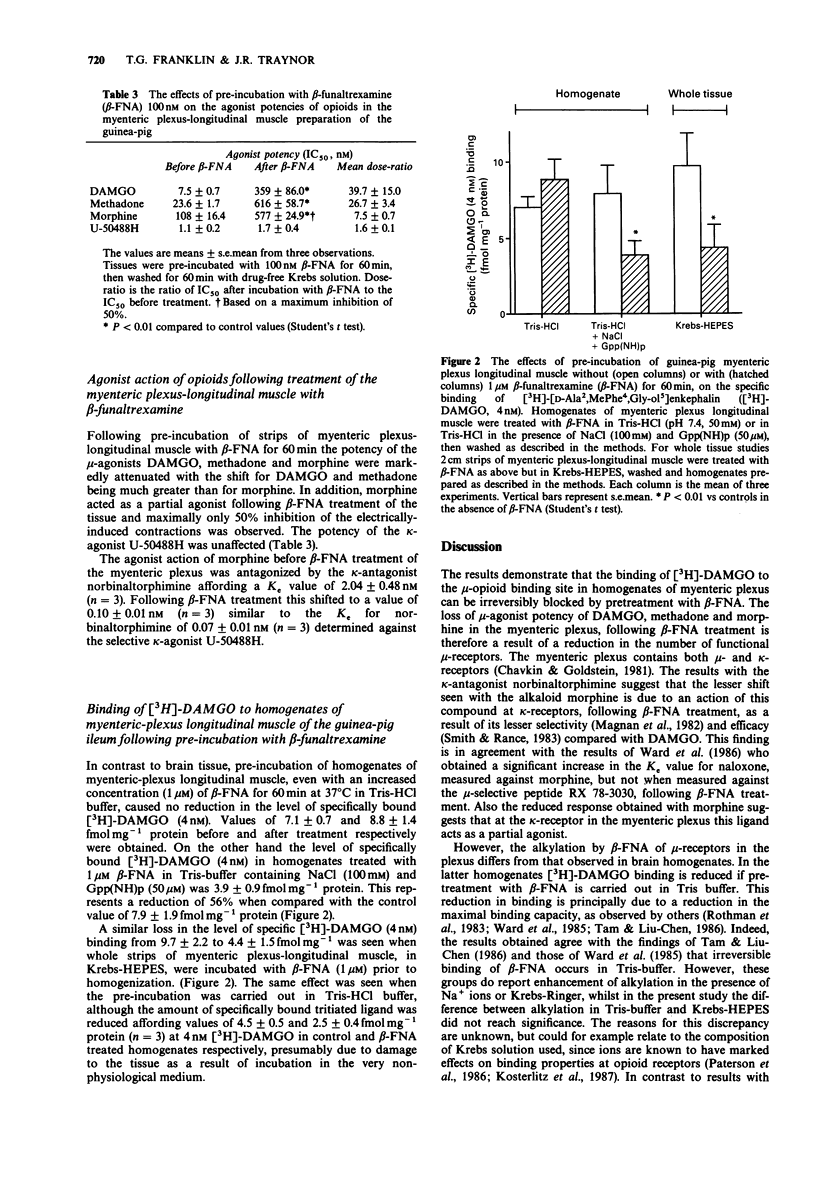
1. The effects of pre-incubation with beta-funaltrexamine (beta-FNA) on the binding of [3H]-[D-Ala2, MePhe4, Gly-ol5]enkephalin ([3H]-DAMGO) to homogenates of guinea-pig brain and myenteric-plexus longitudinal muscle have been studied. 2. beta-FNA pretreatment of brain homogenates in Tris-HCl buffer reduced the amount of [3H]-DAMGO binding. This was principally due to a reduction in the maximal number of binding sites measurable. However, approximately 30% of sites labelled by 1 nM [3H]-DAMGO were insensitive to 1 microM beta-FNA. Similar findings were obtained when the alkylation was performed in brain homogenates prepared in Krebs solution buffered with HEPES. 3. beta-FNA pretreatment of whole myenteric-plexus longitudinal muscle strips caused an increase in the IC50 values of mu-agonists, but not of kappa-agonists. However, the binding of [3H]-DAMGO to homogenates of myenteric-plexus longitudinal muscle was not altered by pre-incubation with beta-FNA in Tris-HCl buffer. On the other hand when the pretreatment was carried out in whole tissue in Krebs solution, or in homogenates in the presence of NaCl and Gpp(NH)p, a marked reduction in [3H]-DAMGO binding was observed. 4. These results suggest that a low affinity form of the mu-opioid receptor is the physiologically relevant site for beta-FNA alkylation in the myenteric-plexus and that differences exist between mu-receptor systems in guinea-pig myenteric plexus and brain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A. J. Interaction of ligands with the opiate receptors of brain membranes: regulation by ions and nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1713–1717. doi: 10.1073/pnas.75.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. A., Shaw J. S., Wickenden A. D. The physiological relevance of low agonist affinity binding at opioid mu-receptors. Br J Pharmacol. 1988 Jun;94(2):625–631. doi: 10.1111/j.1476-5381.1988.tb11569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C., Goldstein A. Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Corbett A. D., Kostelitz H. W., McKnight A. T., Paterson S. J., Robson L. E. Pre-incubation of guinea-pig myenteric plexus with beta-funaltrexamine: discrepancy between binding assays and bioassays. Br J Pharmacol. 1985 Jul;85(3):665–673. doi: 10.1111/j.1476-5381.1985.tb10562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R., Kosterlitz H. W., Paterson S. J., Rance M. J., Traynor J. R. The use of [3H]-[D-Pen2,D-Pen5]enkephalin as a highly selective ligand for the delta-binding site. Br J Pharmacol. 1985 Apr;84(4):927–932. doi: 10.1111/j.1476-5381.1985.tb17387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey E. A., Gosse M. E., Cote T. E. Reconstitution of the solubilized mu-opioid receptor coupled to a GTP-binding protein. Eur J Pharmacol. 1989 Oct 17;172(4-5):347–356. doi: 10.1016/0922-4106(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Gillan M. G., Kosterlitz H. W., Paterson S. J. Comparison of the binding characteristics of tritiated opiates and opioid peptides. Br J Pharmacol. 1980 Nov;70(3):481–490. doi: 10.1111/j.1476-5381.1980.tb08727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Handa B. K., Land A. C., Lord J. A., Morgan B. A., Rance M. J., Smith C. F. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur J Pharmacol. 1981 Apr 9;70(4):531–540. doi: 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- Hayes A. G., Sheehan M. J., Tyers M. B. Determination of the receptor selectivity of opioid agonists in the guinea-pig ileum and mouse vas deferens by use of beta-funaltrexamine. Br J Pharmacol. 1985 Dec;86(4):899–904. doi: 10.1111/j.1476-5381.1985.tb11112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. G., Skingle M., Tyers M. B. Reversal by beta-funaltrexamine of the antinociceptive effect of opioid agonists in the rat. Br J Pharmacol. 1986 Aug;88(4):867–872. doi: 10.1111/j.1476-5381.1986.tb16260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Paterson S. J., Robson L. E. Characterization of the kappa-subtype of the opiate receptor in the guinea-pig brain. Br J Pharmacol. 1981 Aug;73(4):939–949. doi: 10.1111/j.1476-5381.1981.tb08749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Paterson S. J., Robson L. E., Traynor J. R. Effects of cations on binding, in membrane suspensions, of various opioids at mu-sites of rabbit cerebellum and kappa-sites of guinea-pig cerebellum. Br J Pharmacol. 1987 Jun;91(2):431–437. doi: 10.1111/j.1476-5381.1987.tb10298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Watt A. J. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone). Br J Pharmacol Chemother. 1968 Jun;33(2):266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan J., Paterson S. J., Tavani A., Kosterlitz H. W. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):197–205. doi: 10.1007/BF00495865. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Milligan G. Techniques used in the identification and analysis of function of pertussis toxin-sensitive guanine nucleotide binding proteins. Biochem J. 1988 Oct 1;255(1):1–13. doi: 10.1042/bj2550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson S. J., Robson L. E., Kosterlitz H. W. Control by cations of opioid binding in guinea pig brain membranes. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6216–6220. doi: 10.1073/pnas.83.16.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese P. S., Larson D. L., Sayre L. M., Fries D. S., Takemori A. E. A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities. J Med Chem. 1980 Mar;23(3):233–234. doi: 10.1021/jm00177a002. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S., Takemori A. E. Different receptor sites mediate opioid agonism and antagonism. J Med Chem. 1983 Oct;26(10):1341–1343. doi: 10.1021/jm00364a001. [DOI] [PubMed] [Google Scholar]
- Robson L. E., Gillan M. G., Kosterlitz H. W. Species differences in the concentrations and distributions of opioid binding sites. Eur J Pharmacol. 1985 May 28;112(1):65–71. doi: 10.1016/0014-2999(85)90239-0. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Bowen W. D., Schumacher U. K., Pert C. B. Effect of beta-FNA on opiate receptor binding: preliminary evidence for two types of mu receptors. Eur J Pharmacol. 1983 Nov 11;95(1-2):147–148. doi: 10.1016/0014-2999(83)90282-0. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Jacobson A. E., Rice K. C., Herkenham M. Autoradiographic evidence for two classes of mu opioid binding sites in rat brain using [125I]FK33824. Peptides. 1987 Nov-Dec;8(6):1015–1021. doi: 10.1016/0196-9781(87)90130-6. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Hiller J. M., Groth J., Edelman I. Further properties of stereospecific opiate binding sites in rat brain: on the nature of the sodium effect. J Pharmacol Exp Ther. 1975 Mar;192(3):531–537. [PubMed] [Google Scholar]
- Smith C. F., Rance M. J. Opiate receptors in the rat vas deferens. Life Sci. 1983;33 (Suppl 1):327–330. doi: 10.1016/0024-3205(83)90509-x. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Harden T. K. Modification of receptor-mediated inhibition of adenylate cyclase in NG108-15 neuroblastoma X glioma cells by n-ethylmaleimide. J Pharmacol Exp Ther. 1984 Feb;228(2):425–433. [PubMed] [Google Scholar]
- Spain J. W., Coscia C. J. Multiple interconvertible affinity states for the delta opioid agonist-receptor complex. J Biol Chem. 1987 Jul 5;262(19):8948–8951. [PubMed] [Google Scholar]
- Takemori A. E., Portoghese P. S. Evidence for the interaction of morphine with kappa and delta opioid receptors to induce analgesia in beta-funaltrexamine-treated mice. J Pharmacol Exp Ther. 1987 Oct;243(1):91–94. [PubMed] [Google Scholar]
- Takemori A. E., Ward A., Portoghese P. S., Telang V. G. Potential nonequilibrium analgetic receptor inactivators. Further pharmacologic studies of N-acylanileridines. J Med Chem. 1974 Oct;17(10):1051–1054. doi: 10.1021/jm00256a004. [DOI] [PubMed] [Google Scholar]
- Tam S. W., Liu-Chen L. Y. Reversible and irreversible binding of beta-funaltrexamine to mu, delta and kappa opioid receptors in guinea pig brain membranes. J Pharmacol Exp Ther. 1986 Nov;239(2):351–357. [PubMed] [Google Scholar]
- Traynor J. R., Corbett A. D., Kosterlitz H. W. Diprenorphine has agonist activity at opioid kappa-receptors in the myenteric plexus of the guinea-pig ileum. Eur J Pharmacol. 1987 May 7;137(1):85–89. doi: 10.1016/0014-2999(87)90185-3. [DOI] [PubMed] [Google Scholar]
- Ward S. J., Fries D. S., Larson D. L., Portoghese P. S., Takemori A. E. Opioid receptor binding characteristics of the non-equilibrium mu antagonist, beta-funaltrexamine (beta-FNA). Eur J Pharmacol. 1985 Jan 8;107(3):323–330. doi: 10.1016/0014-2999(85)90257-2. [DOI] [PubMed] [Google Scholar]
- Ward S. J., LoPresti D., James D. W. Activity of mu- and delta-selective opioid agonists in the guinea pig ileum preparation: differentiation into peptide and nonpeptide classes with beta-funaltrexamine. J Pharmacol Exp Ther. 1986 Aug;238(2):625–631. [PubMed] [Google Scholar]
- Ward S. J., Portoghese P. S., Takemori A. E. Pharmacological characterization in vivo of the novel opiate, beta-funaltrexamine. J Pharmacol Exp Ther. 1982 Mar;220(3):494–498. [PubMed] [Google Scholar]
- Ward S. J., Portoghese P. S., Takemori A. E. Pharmacological profiles of beta-funaltrexamine (beta-FNA) and beta-chlornaltrexamine (beta-CNA) on the mouse vas deferens preparation. Eur J Pharmacol. 1982 Jun 4;80(4):377–384. doi: 10.1016/0014-2999(82)90083-8. [DOI] [PubMed] [Google Scholar]
- Werling L. L., Puttfarcken P. S., Cox B. M. Multiple agonist-affinity states of opioid receptors: regulation of binding by guanyl nucleotides in guinea pig cortical, NG108-15, and 7315c cell membranes. Mol Pharmacol. 1988 Apr;33(4):423–431. [PubMed] [Google Scholar]
- Wong Y. H., Demoliou-Mason C. D., Barnard E. A. Opioid receptors in magnesium-digitonin-solubilized rat brain membranes are tightly coupled to a pertussis toxin-sensitive guanine nucleotide-binding protein. J Neurochem. 1989 Apr;52(4):999–1009. doi: 10.1111/j.1471-4159.1989.tb01840.x. [DOI] [PubMed] [Google Scholar]


