Abstract
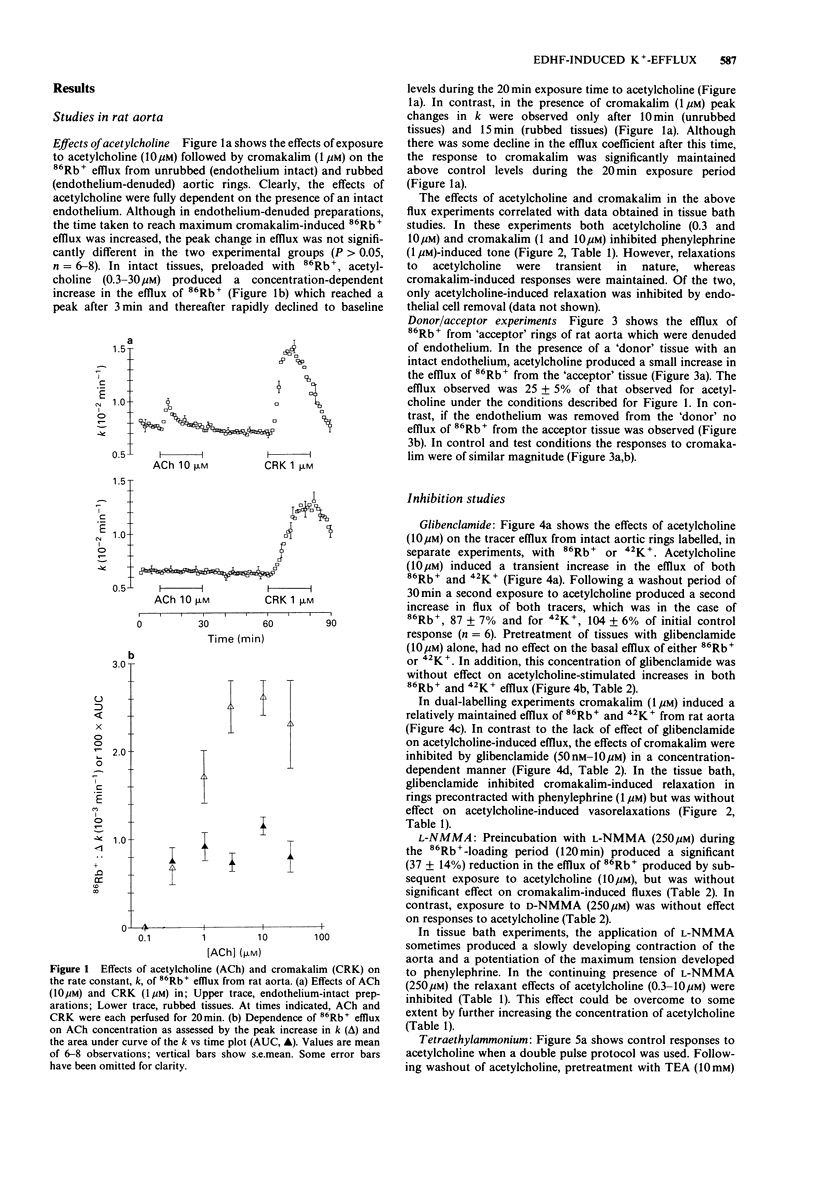
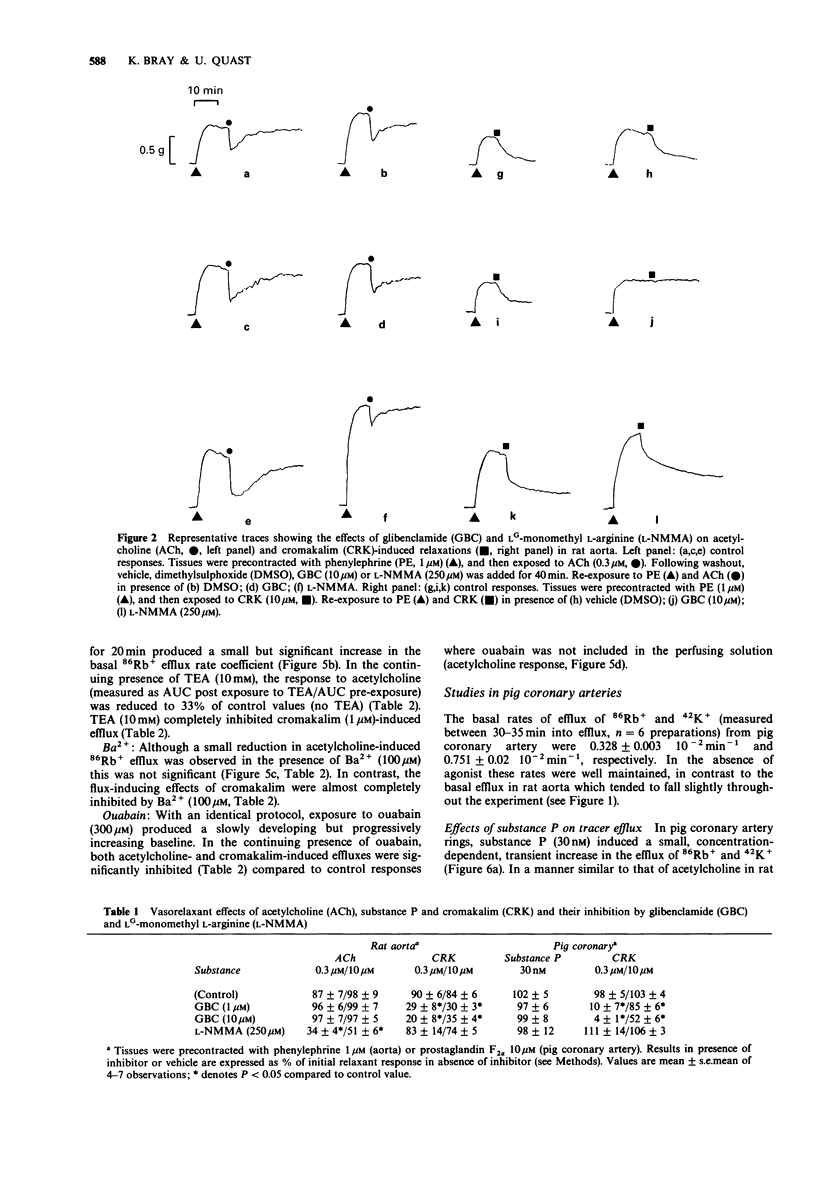
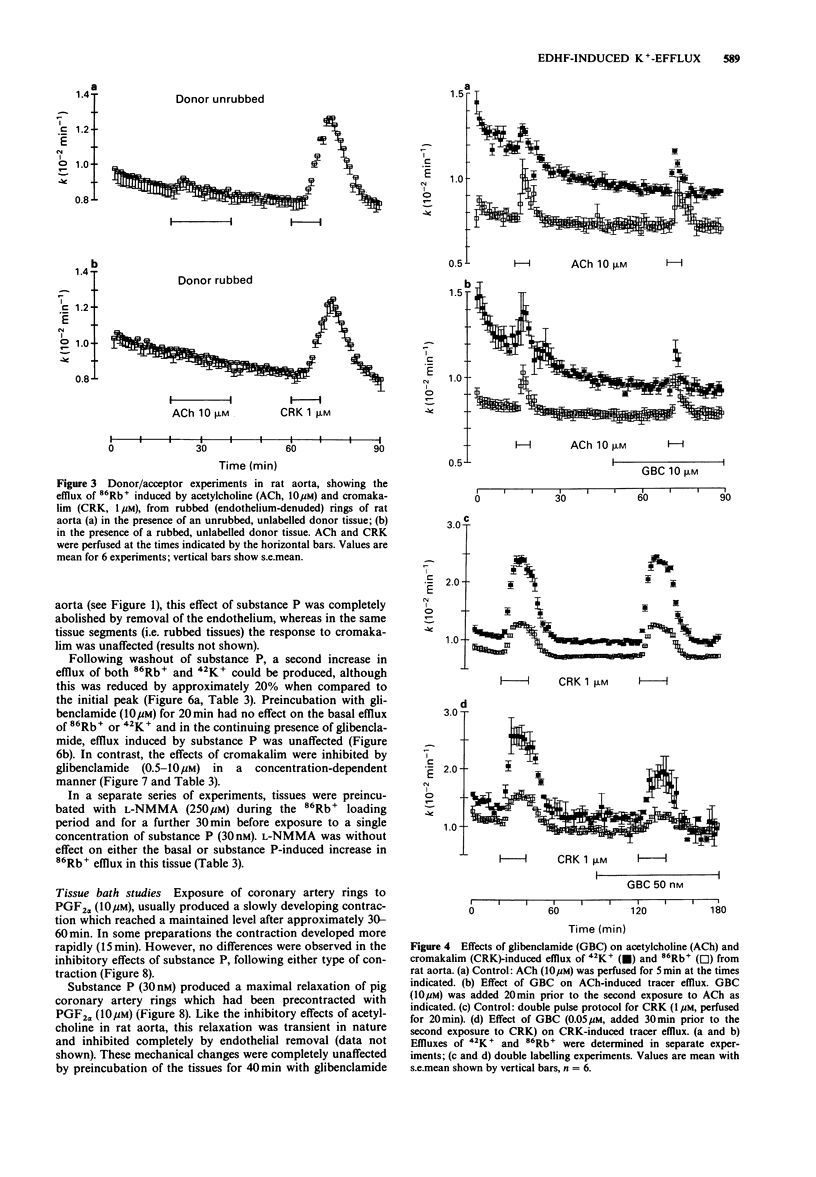
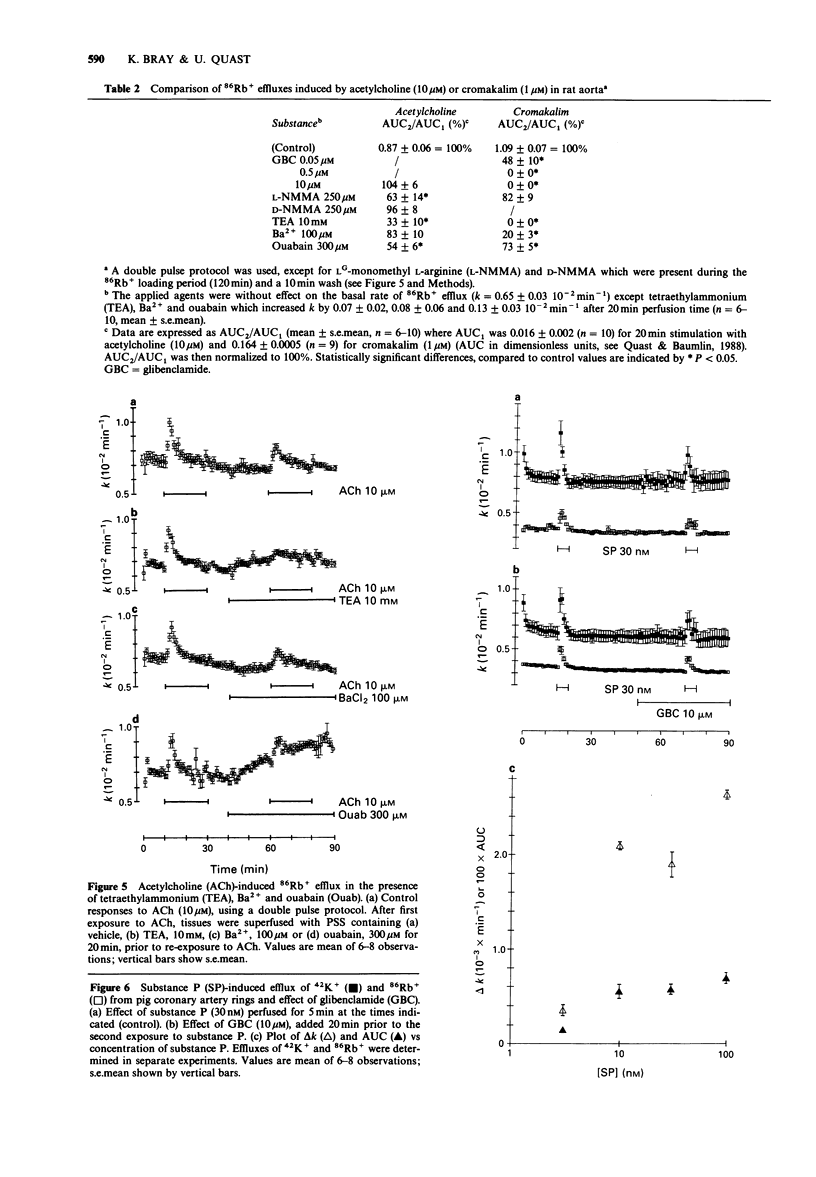
1. The effects of acetylcholine and substance P on the efflux of 86Rb+ and 42K+ from rat aorta and pig coronary artery, respectively, were compared with those of the K+ channel opening agent, cromakalim. 2. In rat aorta preloaded with 86Rb+ and/or 42K+, acetylcholine produced transient, concentration-dependent increases in the efflux rate coefficients of these tracers (maximum approximately 35%). These effects were abolished by endothelial cell removal. 3. Donor/acceptor experiments with rat aorta suggested that at least some of the efflux of 86Rb+ seen in the presence of acetylcholine was not derived from the endothelium, but came from the smooth muscle itself. 4. Acetylcholine (10 microM)-induced 86Rb+ efflux was reduced by tetraethylammonium (TEA, 10 mM) to 33% and ouabain (300 microM) to 54% of control. Preincubation with Ba2+ (100 microM) did not significantly inhibit acetylcholine-induced efflux. 5. Acetylcholine-induced 42K+/86Rb+ efflux was unaffected by preincubation with glibenclamide (10 microM). In contrast, the 42K+/86Rb+ efflux induced by cromakalim was inhibited by glibenclamide (50 nM) by 50%. 6. Acetylcholine (0.3-10 microM)-induced inhibition of phenylephrine (1 microM)-induced tone was abolished by endothelial cell removal but unaffected by glibenclamide. Cromakalim-induced relaxations were endothelium-independent and were inhibited by glibenclamide in a concentration-dependent manner. 7. LG-monomethyl L-arginine (L-NMMA, 250 microM) produced a significant (37 +/- 14%) inhibition of acetylcholine-induced 86Rb+ efflux whereas DG-monomethyl L-arginine was without effect. In the tissue bath L-NMMA inhibited relaxations produced by acetylcholine (0.3-10 microM), but was without effect on responses to cromakalim. 8. In the pig coronary artery, substance P induced an endothelium-dependent efflux of 86Rb+ and 42K+, which was unaffected by preincubation with glibenclamide (10 microM) or L-NMMA (250 microM). 9. The present study shows that acetylcholine and substance P each open K(+)-channels in arterial smooth muscle. However, the insensitivity of the stimulated 86Rb/42K+ efflux to inhibition by glibenclamide suggests that the K(+)-channel opened by these agents is different from the K(+)-channel opened by cromakalim. In addition, the inability of L-NMMA to inhibit fully the acetylcholine- and substance P-stimulated 86Rb+ efflux suggests that in rat aorta and pig coronary artery the endothelium-derived hyperpolarizing factor(s) (EDHF) is different from endothelium-derived relaxing factor (EDRF).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford M. L., Sturgess N. C., Trout N. J., Gardner N. J., Hales C. N. Adenosine-5'-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988 Aug;412(3):297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beny J. L., Brunet P. C., Huggel H. Effect of mechanical stimulation, substance P and vasoactive intestinal polypeptide on the electrical and mechanical activities of circular smooth muscles from pig coronary arteries contracted with acetylcholine: role of endothelium. Pharmacology. 1986;33(2):61–68. doi: 10.1159/000138202. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Lategan T. W. Na-Ca exchange in vascular smooth muscle. J Hypertens. 1985 Apr;3(2):109–116. doi: 10.1097/00004872-198504000-00002. [DOI] [PubMed] [Google Scholar]
- Brunet P. C., Bény J. L. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26(4):228–234. doi: 10.1159/000158770. [DOI] [PubMed] [Google Scholar]
- Buckingham R. E., Hamilton T. C., Howlett D. R., Mootoo S., Wilson C. Inhibition by glibenclamide of the vasorelaxant action of cromakalim in the rat. Br J Pharmacol. 1989 May;97(1):57–64. doi: 10.1111/j.1476-5381.1989.tb11923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavero I., Mondot S., Mestre M. Vasorelaxant effects of cromakalim in rats are mediated by glibenclamide-sensitive potassium channels. J Pharmacol Exp Ther. 1989 Mar;248(3):1261–1268. [PubMed] [Google Scholar]
- Chen G., Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol. 1989 Mar;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldwell M. C., Howlett D. R. Potassium efflux enhancement by cromakalim (BRL 34915) in rabbit mesenteric artery: an indirect effect independent of calcium? Biochem Pharmacol. 1988 Nov 1;37(21):4105–4110. doi: 10.1016/0006-2952(88)90102-5. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Interaction between Na+,K+ exchanges and the direct inhibitory effect of acetylcholine on canine femoral arteries. Circ Res. 1980 Jun;46(6):826–836. doi: 10.1161/01.res.46.6.826. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming W. W. The electrogenic Na+, K+-pump in smooth muscle: physiologic and pharmacologic significance. Annu Rev Pharmacol Toxicol. 1980;20:129–149. doi: 10.1146/annurev.pa.20.040180.001021. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. L., Kim H. S., Okolie P., Weiss G. B. Alterations by glyburide of effects of BRL 34915 and P 1060 on contraction, 86Rb efflux and the maxi-K+ channel in rat portal vein. J Pharmacol Exp Ther. 1990 May;253(2):771–777. [PubMed] [Google Scholar]
- Huang A. H., Busse R., Bassenge E. Endothelium-dependent hyperpolarization of smooth muscle cells in rabbit femoral arteries is not mediated by EDRF (nitric oxide). Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):438–442. doi: 10.1007/BF00172124. [DOI] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Quast U., Baumlin Y. Comparison of the effluxes of 42K+ and 86Rb+ elicited by cromakalim (BRL 34915) in tonic and phasic vascular tissue. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):319–326. doi: 10.1007/BF00173407. [DOI] [PubMed] [Google Scholar]
- Quast U., Cook N. S. In vitro and in vivo comparison of two K+ channel openers, diazoxide and cromakalim, and their inhibition by glibenclamide. J Pharmacol Exp Ther. 1989 Jul;250(1):261–271. [PubMed] [Google Scholar]
- Quast U., Cook N. S. Moving together: K+ channel openers and ATP-sensitive K+ channels. Trends Pharmacol Sci. 1989 Nov;10(11):431–435. doi: 10.1016/S0165-6147(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Schwartz K., Murad F. Effects of Na+,K+-pump inhibitors and membrane depolarizing agents on acetylcholine-induced endothelium-dependent relaxation and cyclic GMP accumulation in rat aorta. Eur J Pharmacol. 1985 Apr 2;110(2):203–209. doi: 10.1016/0014-2999(85)90212-2. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985 Aug 22;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Kozlowski R. Z., Carrington C. A., Hales C. N., Ashford M. L. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol. 1988 Sep;95(1):83–94. doi: 10.1111/j.1476-5381.1988.tb16551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Southerton J. S., Weston A. H., Baker J. R. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol. 1988 Jul;94(3):853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- XIth International Congress of Pharmacology. Amsterdam, The Netherlands, Wednesday 4 July, 1990. Brief communications. Eur J Pharmacol. 1990 Jul;183(4):1123–1616. [PubMed] [Google Scholar]


