Abstract
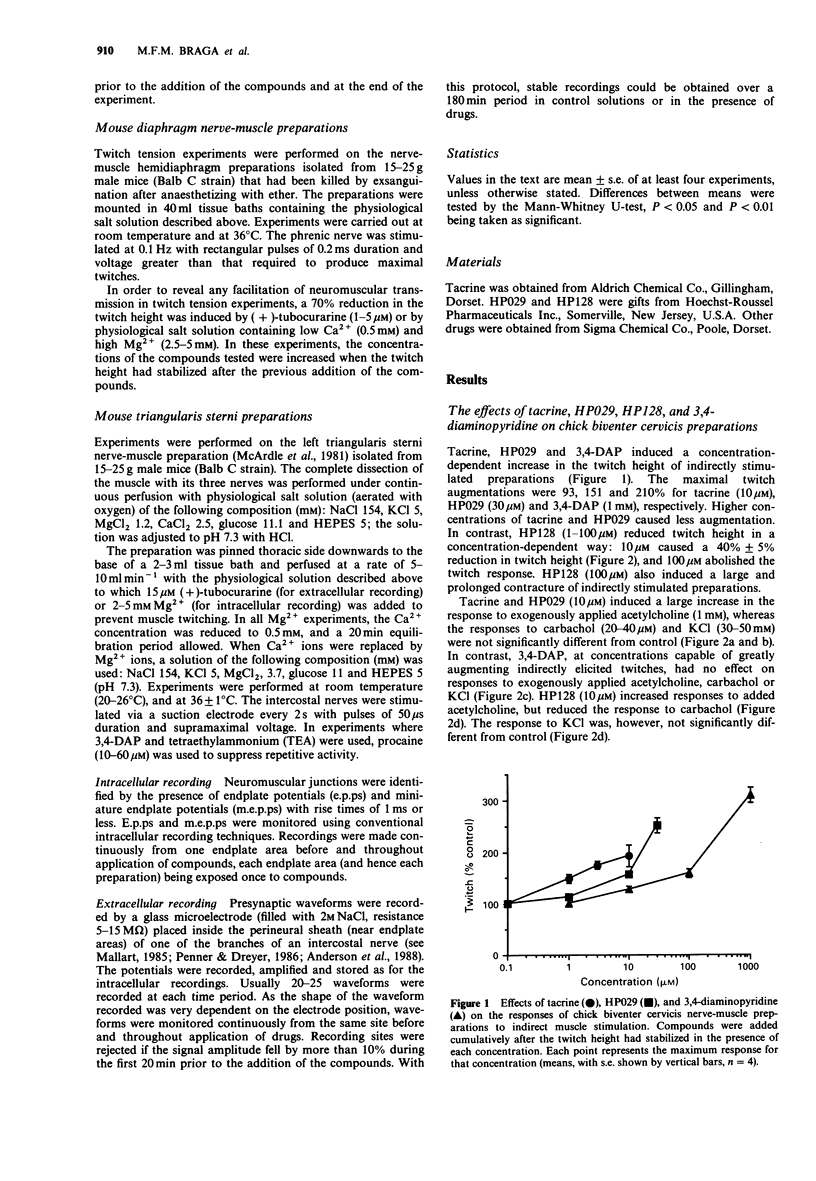
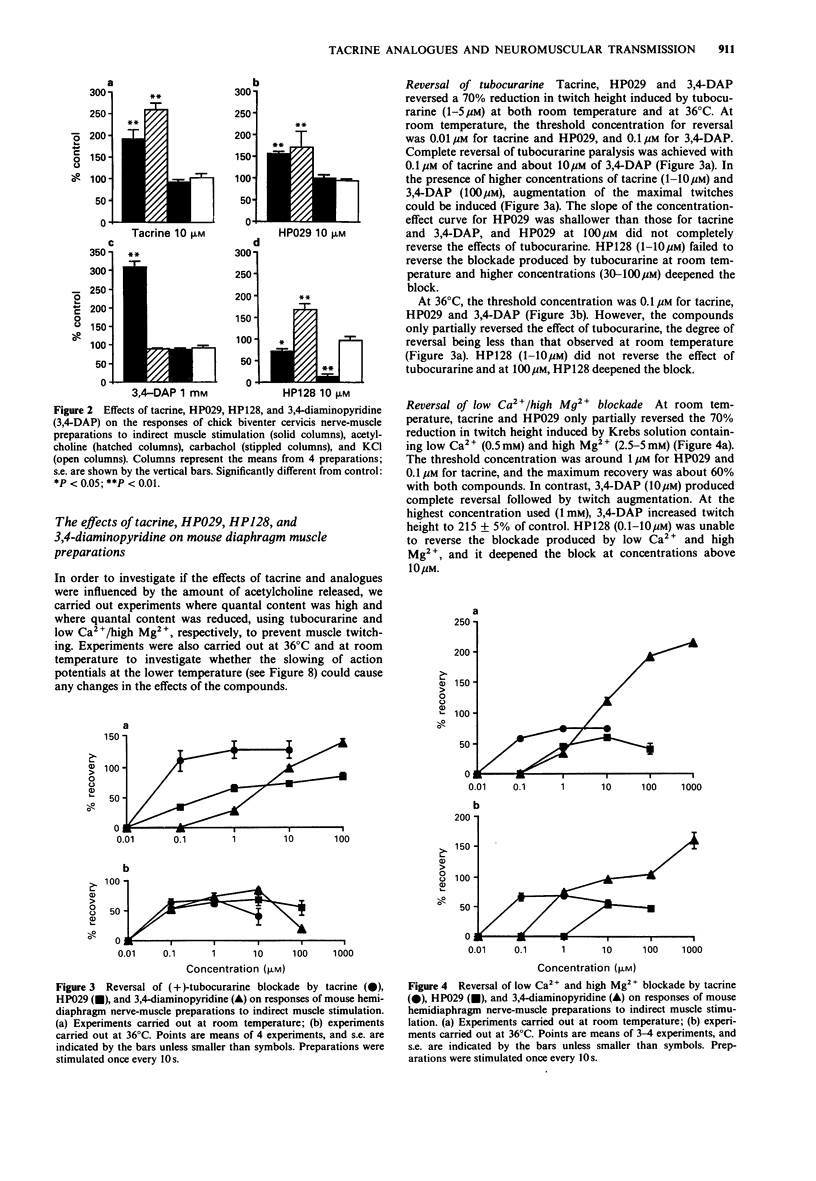
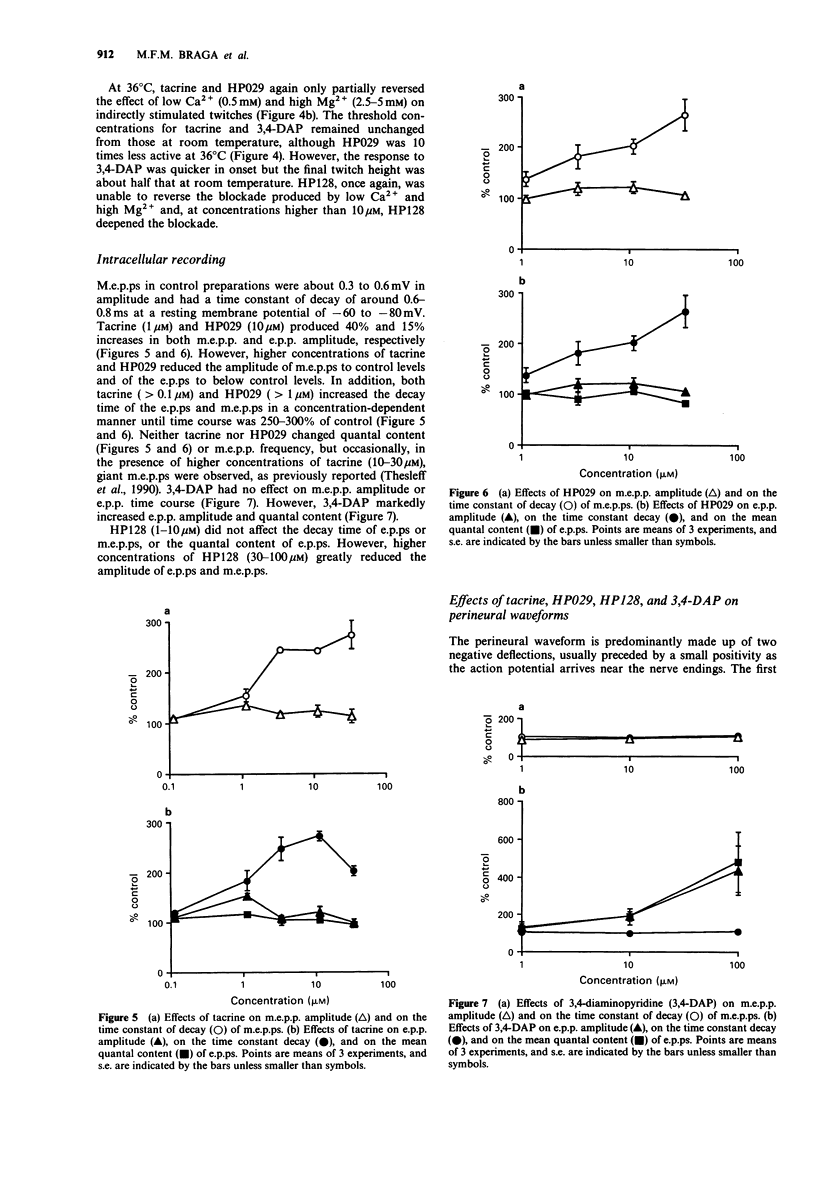
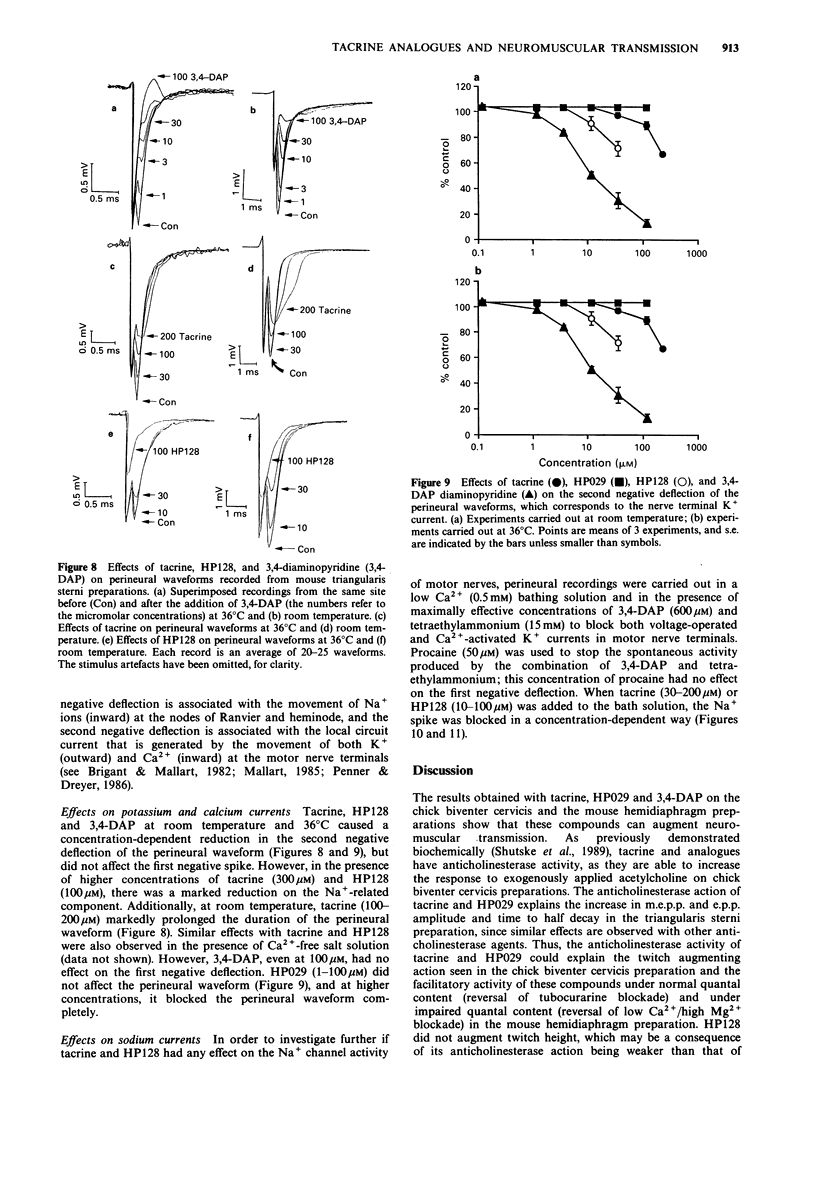
1. The effects of tacrine (9-amino-1,2,3,4-tetrahydroacridine), velnacrine (HP029, 9-amino-1,2,3,4-tetrahydroacridin-1-ol maleate), suronacrine (HP128, 9-benzylamino-1,2,3,4-tetrahydroacridin-1-ol maleate), and 3,4-diaminopyridine on neuromuscular transmission were compared on isolated nerve-muscle preparations. 2. Tacrine, HP029, and 3,4-diaminopyridine augmented responses of chick biventer cervicis preparations to nerve stimulation, with tacrine and HP029 increasing responses to exogenously applied acetylcholine. HP128 blocked responses to nerve stimulation and to carbachol, but increased responses to acetylcholine. 3. In mouse diaphragm preparations that were partially paralysed by tubocurarine or low calcium solutions, tacrine, HP029, and 3,4-diaminopyridine reversed the twitch block. HP128 deepened the block. 4. In mouse triangularis sterni preparations, tacrine and HP029 prolonged the decay phase of endplate potentials and miniature endplate potentials, but had no effect on quantal content at 36 degrees C; above 10 microM, they reduced endplate potential amplitude. 3,4-Diaminopyridine increased quantal content without affecting the time course of the endplate potentials. HP128 (1-10 microM) had no effect on amplitude or time course of endplate potentials, but reduced their amplitude at higher concentrations. 5. Extracellular recording of nerve terminal currents from triangularis sterni preparations revealed that 3,4-diaminopyridine and HP128 had a selective blocking action on the waveform associated with K+ currents, tacrine reduced and prolonged the K(+)-related waveform, and HP029 had nonselective blocking actions only seen at high concentrations. 6. Tacrine and HP029 behave predominantly as anticholinesterase agents, while HP128 has weaker anticholinesterase actions that are masked by cholinoceptor blockade. Tacrine and HP128, but not HP029, have some blocking actions on K+ currents of mouse motor nerve terminals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames D. J., Bhathal P. S., Davies B. M., Fraser J. R. Hepatotoxicity of tetrahydroaminoacridine. Lancet. 1988 Apr 16;1(8590):887–887. doi: 10.1016/s0140-6736(88)91636-4. [DOI] [PubMed] [Google Scholar]
- Anderson A. J., Harvey A. L., Rowan E. G., Strong P. N. Effects of charybdotoxin, a blocker of Ca2+-activated K+ channels, on motor nerve terminals. Br J Pharmacol. 1988 Dec;95(4):1329–1335. doi: 10.1111/j.1476-5381.1988.tb11772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukarch B., Kits K. S., Van der Meer E. G., Lodder J. C., Stoof J. C. 9-Amino-1,2,3,4-tetrahydroacridine (THA), an alleged drug for the treatment of Alzheimer's disease, inhibits acetylcholinesterase activity and slow outward K+ current. Eur J Pharmacol. 1987 Sep 2;141(1):153–157. doi: 10.1016/0014-2999(87)90424-9. [DOI] [PubMed] [Google Scholar]
- Durant N. N., Marshall I. G. The effects of 3,4-diaminopyridine on acetylcholine release at the frog neuromuscular junction. Eur J Pharmacol. 1980 Oct 17;67(2-3):201–208. doi: 10.1016/0014-2999(80)90499-9. [DOI] [PubMed] [Google Scholar]
- Elinder F., Mohammed A. K., Winblad B., Arhem P. Effects of THA on ionic currents in myelinated axons of Xenopus laevis. Eur J Pharmacol. 1989 May 30;164(3):599–602. doi: 10.1016/0014-2999(89)90272-0. [DOI] [PubMed] [Google Scholar]
- GINSBORG B. L., WARRINER J. The isolated chick biventer cervicis nerve-muscle preparation. Br J Pharmacol Chemother. 1960 Sep;15:410–411. doi: 10.1111/j.1476-5381.1960.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Grove E. A. 9-Amino-1,2,3,4-tetrahydroacridine (THA) blocks agonist-induced potassium conductance in rat hippocampal neurones. Eur J Pharmacol. 1989 Apr 25;163(2-3):369–372. doi: 10.1016/0014-2999(89)90209-4. [DOI] [PubMed] [Google Scholar]
- Harvey A. L., Marshall I. G. The actions of three diaminopyridines on the chick biventer cervicis muscle. Eur J Pharmacol. 1977 Aug 15;44(4):303–309. doi: 10.1016/0014-2999(77)90303-x. [DOI] [PubMed] [Google Scholar]
- Harvey A. L., Marshall I. G. The facilitatory actions of aminopyridines and tetraethylammonium on neuromuscular transmission and muscle contractility in avian muscle. Naunyn Schmiedebergs Arch Pharmacol. 1977 Aug;299(1):53–60. doi: 10.1007/BF00508637. [DOI] [PubMed] [Google Scholar]
- Harvey A. L., Rowan E. G. Effects of tacrine, aminopyridines, and physostigmine on acetylcholinesterase, acetylcholine release, and potassium currents. Adv Neurol. 1990;51:227–233. [PubMed] [Google Scholar]
- Mallart A. Electric current flow inside perineurial sheaths of mouse motor nerves. J Physiol. 1985 Nov;368:565–575. doi: 10.1113/jphysiol.1985.sp015876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. Alzheimer's drug trial put on hold. Science. 1987 Nov 20;238(4830):1041–1042. doi: 10.1126/science.3317822. [DOI] [PubMed] [Google Scholar]
- McArdle J. J., Angaut-Petit D., Mallart A., Bournaud R., Faille L., Brigant J. L. Advantages of the triangularis sterni muscle of the mouse for investigations of synaptic phenomena. J Neurosci Methods. 1981 Aug;4(2):109–115. doi: 10.1016/0165-0270(81)90044-3. [DOI] [PubMed] [Google Scholar]
- Molgó J., Lundh H., Thesleff S. Potency of 3,4-diaminopyridine and 4-aminopyridine on mammalian neuromuscular transmission and the effect of pH changes. Eur J Pharmacol. 1980 Jan 11;61(1):25–34. doi: 10.1016/0014-2999(80)90378-7. [DOI] [PubMed] [Google Scholar]
- Penner R., Dreyer F. Two different presynaptic calcium currents in mouse motor nerve terminals. Pflugers Arch. 1986 Feb;406(2):190–197. doi: 10.1007/BF00586682. [DOI] [PubMed] [Google Scholar]
- Puri S. K., Hsu R. S., Ho I., Lassman H. B. Single dose safety, tolerance, and pharmacokinetics of HP 029 in healthy young men: a potential Alzheimer agent. J Clin Pharmacol. 1989 Mar;29(3):278–284. doi: 10.1002/j.1552-4604.1989.tb03328.x. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A. Tetrahydroaminoacridine blocks voltage-dependent ion channels in hippocampal neurons. Eur J Pharmacol. 1987 Oct 6;142(1):169–172. doi: 10.1016/0014-2999(87)90670-4. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Sattin A. Tetrahydroaminoacridine blocks potassium channels and inhibits sodium inactivation in Myxicola. J Pharmacol Exp Ther. 1987 Nov;243(2):609–613. [PubMed] [Google Scholar]
- Shutske G. M., Pierrat F. A., Cornfeldt M. L., Szewczak M. R., Huger F. P., Bores G. M., Haroutunian V., Davis K. L. (+/-)-9-Amino-1,2,3,4-tetrahydroacridin-1-ol. A potential Alzheimer's disease therapeutic of low toxicity. J Med Chem. 1988 Jul;31(7):1278–1279. doi: 10.1021/jm00402a002. [DOI] [PubMed] [Google Scholar]
- Shutske G. M., Pierrat F. A., Kapples K. J., Cornfeldt M. L., Szewczak M. R., Huger F. P., Bores G. M., Haroutunian V., Davis K. L. 9-Amino-1,2,3,4-tetrahydroacridin-1-ols: synthesis and evaluation as potential Alzheimer's disease therapeutics. J Med Chem. 1989 Aug;32(8):1805–1813. doi: 10.1021/jm00128a024. [DOI] [PubMed] [Google Scholar]
- Stevens D. R., Cotman C. W. Excitatory actions of tetrahydro-9-aminoacridine (THA) on hippocampal pyramidal neurons. Neurosci Lett. 1987 Aug 31;79(3):301–305. doi: 10.1016/0304-3940(87)90448-4. [DOI] [PubMed] [Google Scholar]
- Summers W. K., Majovski L. V., Marsh G. M., Tachiki K., Kling A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med. 1986 Nov 13;315(20):1241–1245. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]
- Thesleff S., Sellin L. C., Tågerud S. Tetrahydroaminoacridine (tacrine) stimulates neurosecretion at mammalian motor endplates. Br J Pharmacol. 1990 Jul;100(3):487–490. doi: 10.1111/j.1476-5381.1990.tb15834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


