Abstract
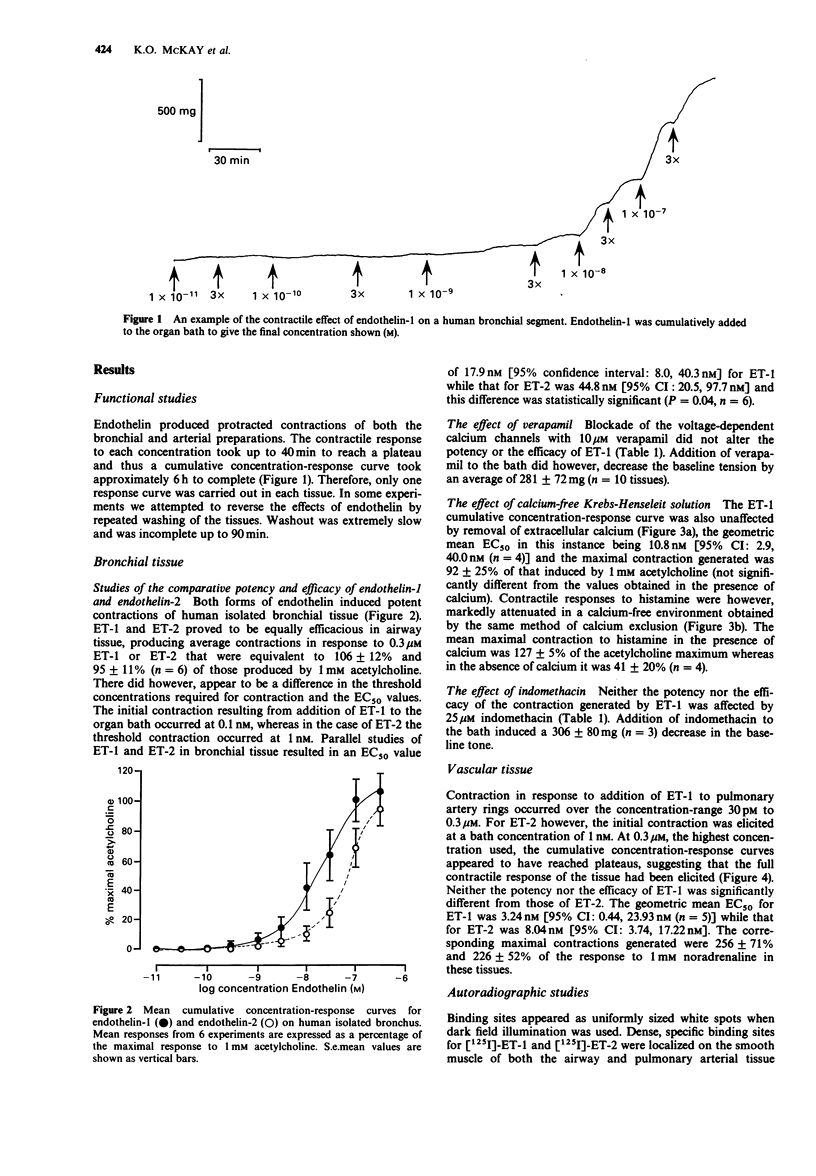
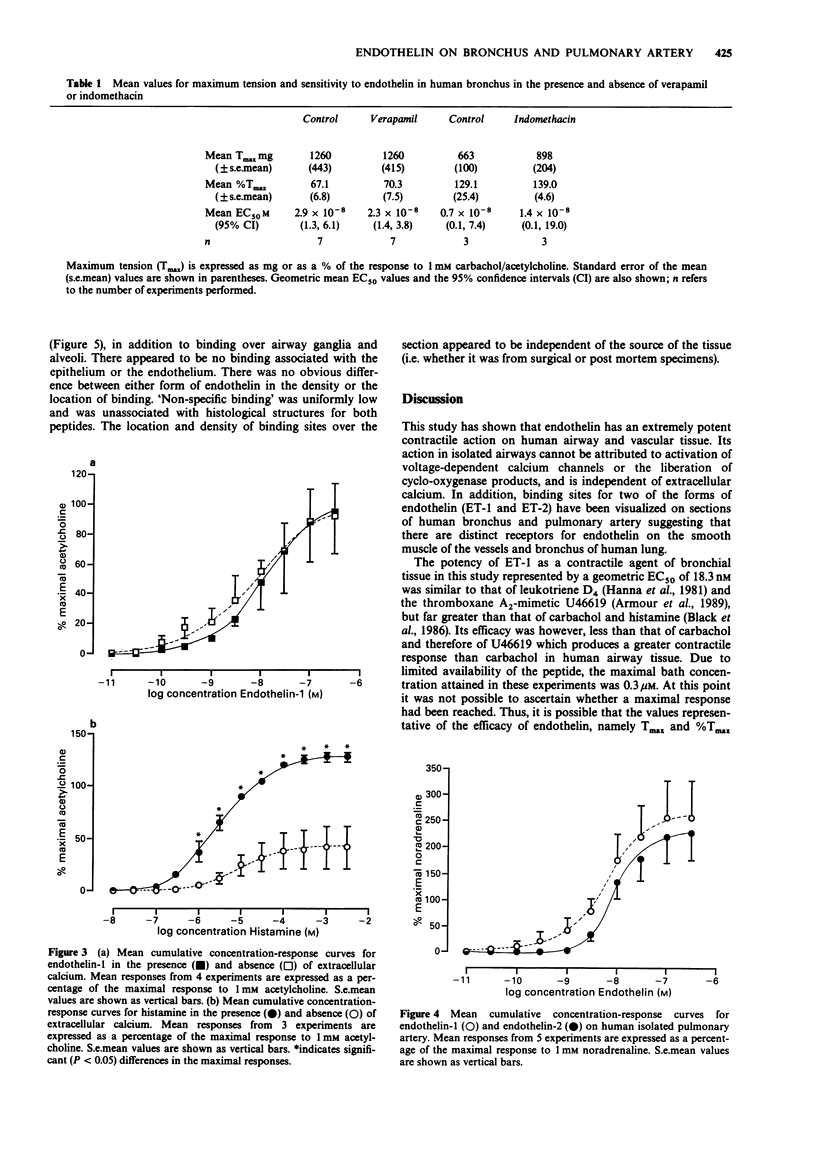
1 The peptides endothelin-1 (ET-1) and endothelin-2 (ET-2) elicited potent and sustained contractions of human isolated bronchus and pulmonary artery. 2 ET-1 is one of the most potent contractile agonists investigated in these tissues with an EC50 value of 18.3 nM (95% confidence interval: 12.9, 25.9 nM: n = 26) in bronchus and 3.2 nM (95% confidence interval: 0.4, 23.9 nM; n = 5) in the arterial preparation. 3 ET-1 is 2.5 times more potent than ET-2 in both the airway and vascular tissues, and both forms of the peptide have geometric mean EC50 values 5 times greater than in the isolated bronchial tissue than in the pulmonary artery. 4 Neither pretreatment with the voltage-dependent calcium (VDC) channel antagonist verapamil (10 microM) nor with indomethacin (25 microM) significantly altered the response curve to ET-1 in human isolated bronchus. Removal of calcium from the Krebs-Henseleit solution did not affect ET-1-induced responses. 5 Specific binding on the smooth muscle of human airway and pulmonary arterial tissue to both ET-1 and ET-2 was detected in autoradiographic studies. There appeared to be no difference between the peptides in the location nor the density of binding sites. 6 We conclude that contraction of human bronchial tissue by ET-1 is not dependent upon influence of extracellular calcium nor release of prostaglandins or thromboxane A2. It is likely that the action of ET-1 in this tissue is due to binding of this peptide to specific receptors located on the smooth muscle.
Full text
PDF



Images in this article
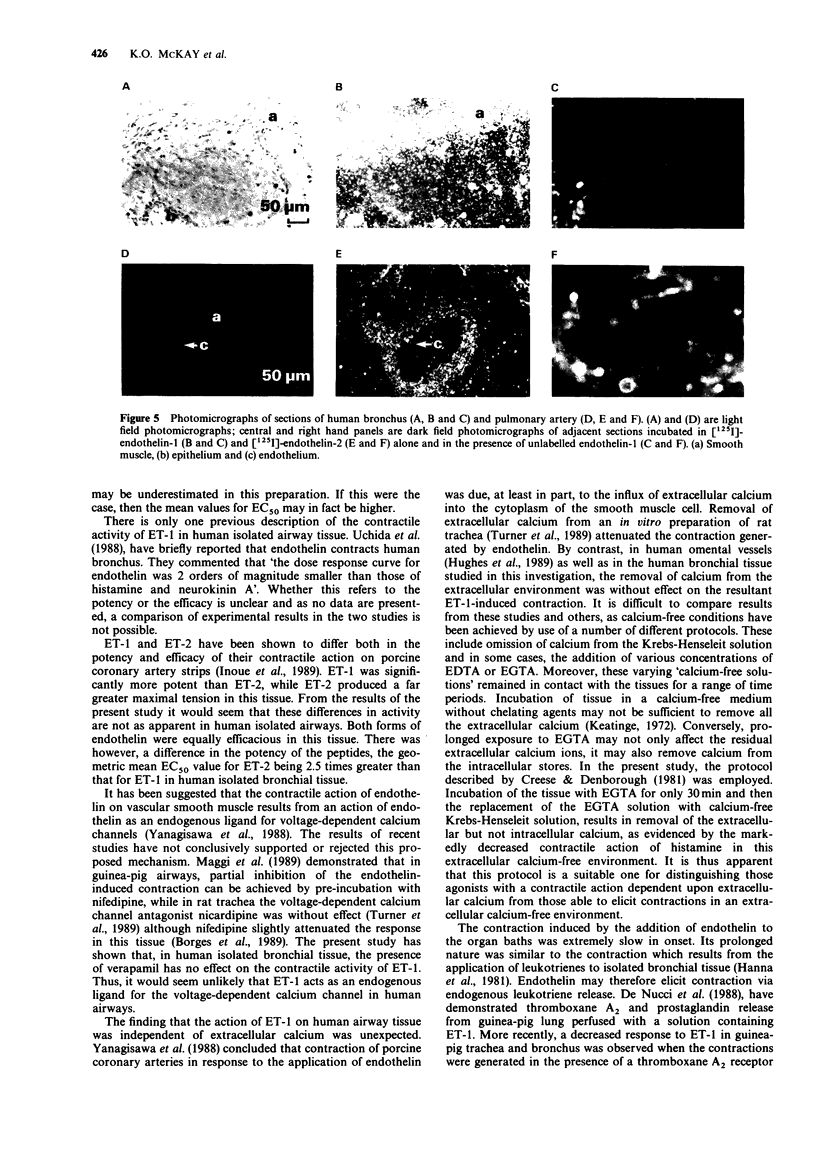
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armour C. L., Black J. L., Johnson P. R., Vincenc K. S., Berend N. Formyl peptide-induced contraction of human airways in vitro. J Appl Physiol (1985) 1986 Jan;60(1):141–146. doi: 10.1152/jappl.1986.60.1.141. [DOI] [PubMed] [Google Scholar]
- Armour C. L., Diment L. M., Black J. L. Relationship between smooth muscle volume and contractile response in airway tissue. Isometric versus isotonic measurement. J Pharmacol Exp Ther. 1988 May;245(2):687–691. [PubMed] [Google Scholar]
- Armour C. L., Johnson P. R., Alfredson M. L., Black J. L. Characterization of contractile prostanoid receptors on human airway smooth muscle. Eur J Pharmacol. 1989 Jun 20;165(2-3):215–222. doi: 10.1016/0014-2999(89)90715-2. [DOI] [PubMed] [Google Scholar]
- Battistini B., Filep J., Sirois P. Potent thromboxane-mediated in vitro bronchoconstrictor effect of endothelin in the guinea-pig. Eur J Pharmacol. 1990 Mar 13;178(1):141–142. doi: 10.1016/0014-2999(90)94808-b. [DOI] [PubMed] [Google Scholar]
- Black J., Armour C., Johnson P., Vincenc K. The calcium dependence of histamine, carbachol and potassium chloride-induced contraction in human airways in vitro. Eur J Pharmacol. 1986 Jun 17;125(2):159–168. doi: 10.1016/0014-2999(86)90023-3. [DOI] [PubMed] [Google Scholar]
- Borges R., Von Grafenstein H., Knight D. E. Tissue selectivity of endothelin. Eur J Pharmacol. 1989 Jun 20;165(2-3):223–230. doi: 10.1016/0014-2999(89)90716-4. [DOI] [PubMed] [Google Scholar]
- Creese B. R., Denborough M. A. Sources of calcium for contraction of guinea-pig isolated tracheal smooth muscle. Clin Exp Pharmacol Physiol. 1981 Mar-Apr;8(2):175–182. doi: 10.1111/j.1440-1681.1981.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Dashwood M., Turner M., Jacobs M. Endothelin-1: contractile responses and autoradiographic localization of receptors in rabbit blood vessels. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S183–S185. [PubMed] [Google Scholar]
- Davenport A. P., Nunez D. J., Hall J. A., Kaumann A. J., Brown M. J. Autoradiographical localization of binding sites for porcine [125I]endothelin-1 in humans, pigs, and rats: functional relevance in humans. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S166–S170. doi: 10.1097/00005344-198900135-00045. [DOI] [PubMed] [Google Scholar]
- Fyhrquist F., Saijonmaa O., Metsärinne K., Tikkanen I., Rosenlöf K., Tikkanen T. Raised plasma endothelin-I concentration following cold pressor test. Biochem Biophys Res Commun. 1990 May 31;169(1):217–221. doi: 10.1016/0006-291x(90)91456-3. [DOI] [PubMed] [Google Scholar]
- Hanna C. J., Bach M. K., Pare P. D., Schellenberg R. R. Slow-reacting substances (leukotrienes) contract human airway and pulmonary vascular smooth muscle in vitro. Nature. 1981 Mar 26;290(5804):343–344. doi: 10.1038/290343a0. [DOI] [PubMed] [Google Scholar]
- Hiley C. R. Functional studies on endothelin catch up with molecular biology. Trends Pharmacol Sci. 1989 Feb;10(2):47–49. doi: 10.1016/0165-6147(89)90072-2. [DOI] [PubMed] [Google Scholar]
- Hughes A. D., Thom S. A., Woodall N., Schachter M., Hair W. M., Martin G. N., Sever P. S. Human vascular responses to endothelin-1: observations in vivo and in vitro. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S225–S228. [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Ca concentration and flux in Ca-deprived arteries. J Physiol. 1972 Jul;224(1):35–59. doi: 10.1113/jphysiol.1972.sp009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohzuki M., Johnston C. I., Chai S. Y., Casley D. J., Rogerson F., Mendelsohn F. A. Endothelin receptors in rat adrenal gland visualized by quantitative autoradiography. Clin Exp Pharmacol Physiol. 1989 Apr;16(4):239–242. doi: 10.1111/j.1440-1681.1989.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Patacchini R., Santicioli P., Giachetti A., Meli A. Further studies on the response of the guinea-pig isolated bronchus to endothelins and sarafotoxin S6b. Eur J Pharmacol. 1990 Jan 25;176(1):1–9. doi: 10.1016/0014-2999(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Giuliani S., Meli A. Potent contractile effect of endothelin in isolated guinea-pig airways. Eur J Pharmacol. 1989 Jan 24;160(1):179–182. doi: 10.1016/0014-2999(89)90670-5. [DOI] [PubMed] [Google Scholar]
- Nomura A., Uchida Y., Kameyana M., Saotome M., Oki K., Hasegawa S. Endothelin and bronchial asthma. Lancet. 1989 Sep 23;2(8665):747–748. doi: 10.1016/s0140-6736(89)90814-3. [DOI] [PubMed] [Google Scholar]
- Power R. F., Wharton J., Zhao Y., Bloom S. R., Polak J. M. Autoradiographic localization of endothelin-1 binding sites in the cardiovascular and respiratory systems. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S50–S74. doi: 10.1097/00005344-198900135-00013. [DOI] [PubMed] [Google Scholar]
- Turner N. C., Dollery C. T., Williams A. J. Endothelin-1-induced contractions of vascular and tracheal smooth muscle: effects of nicardipine and BRL 34915. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S180–S182. doi: 10.1097/00005344-198900135-00049. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Ninomiya H., Saotome M., Nomura A., Ohtsuka M., Yanagisawa M., Goto K., Masaki T., Hasegawa S. Endothelin, a novel vasoconstrictor peptide, as potent bronchoconstrictor. Eur J Pharmacol. 1988 Sep 13;154(2):227–228. doi: 10.1016/0014-2999(88)90106-9. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]



