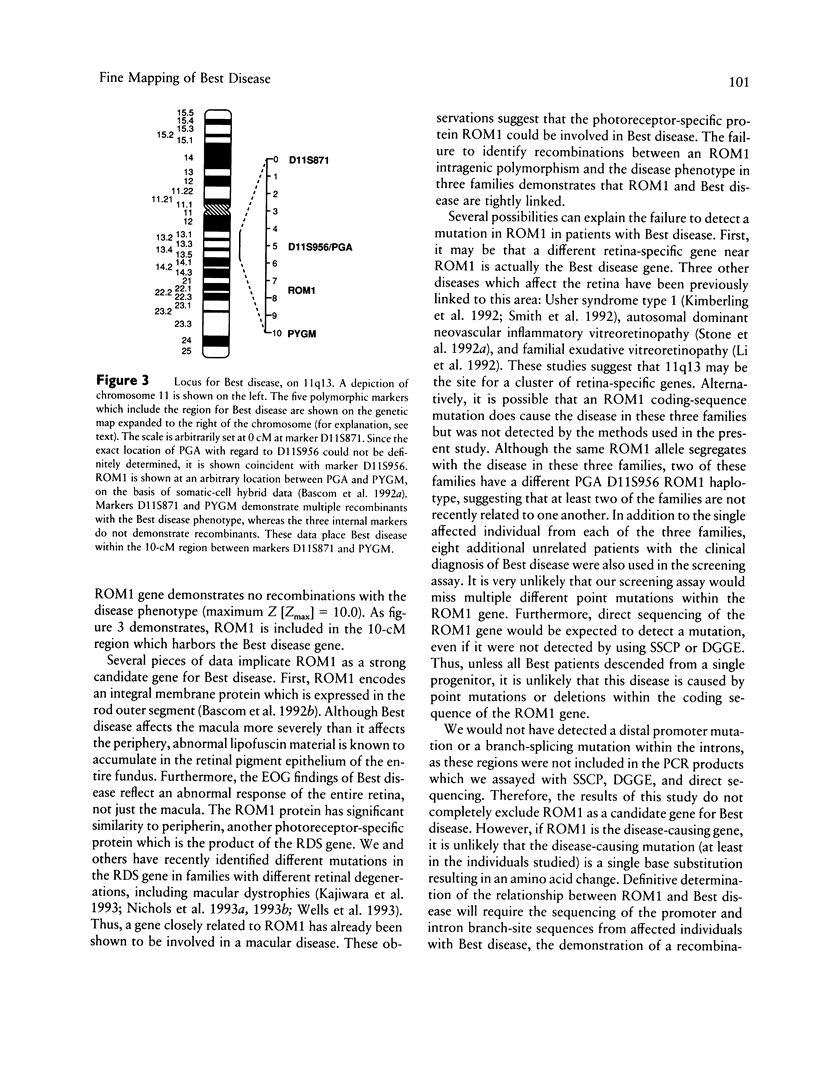
Abstract
Vitelliform macular dystrophy (Best disease) is an autosomal dominant macular dystrophy which shares important clinical features with age-related macular degeneration, the most common cause of legal blindness in the elderly. Unfortunately, our understanding and treatment for this common age-related disorder is limited. Discovery of the gene which causes Best disease has the potential to increase our understanding of the pathogenesis of all types of macular degeneration, including the common age-related form. Best disease has recently been mapped to chromosome 11q13. The photoreceptor-specific protein ROM1 has also been recently mapped to this location, and the ROM1 gene is a candidate gene for Best disease. Using highly polymorphic markers, we have narrowed the genetic region which contains the Best disease gene to the 10-cM region between markers D11S871 and PYGM. Marker D11S956 demonstrated no recombinants with Best disease in three large families and resulted in a lod score of 18.2. In addition, a polymorphism within the ROM1 gene also demonstrated no recombinants and resulted in a lod score of 10.0 in these same three families. We used a combination of SSCP analysis, denaturing gradient gel electrophoresis, and DNA sequencing to screen the entire coding region of the ROM1 gene in 11 different unrelated patients affected with Best disease. No nucleotide changes were found in the coding sequence of any affected patient, indicating that mutations within the coding sequence are unlikely to cause Best disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden G. B., Barrada A., Kelsey J. H. New clinical test of retinal function based upon the standing potential of the eye. Br J Ophthalmol. 1962 Aug;46(8):449–467. doi: 10.1136/bjo.46.8.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom R. A., García-Heras J., Hsieh C. L., Gerhard D. S., Jones C., Francke U., Willard H. F., Ledbetter D. H., McInnes R. R. Localization of the photoreceptor gene ROM1 to human chromosome 11 and mouse chromosome 19: sublocalization to human 11q13 between PGA and PYGM. Am J Hum Genet. 1992 Nov;51(5):1028–1035. [PMC free article] [PubMed] [Google Scholar]
- Bascom R. A., Manara S., Collins L., Molday R. S., Kalnins V. I., McInnes R. R. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992 Jun;8(6):1171–1184. doi: 10.1016/0896-6273(92)90137-3. [DOI] [PubMed] [Google Scholar]
- Bascom R. A., Schappert K., McInnes R. R. Cloning of the human and murine ROM1 genes: genomic organization and sequence conservation. Hum Mol Genet. 1993 Apr;2(4):385–391. doi: 10.1093/hmg/2.4.385. [DOI] [PubMed] [Google Scholar]
- Bassam B. J., Caetano-Anollés G., Gresshoff P. M. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991 Jul;196(1):80–83. doi: 10.1016/0003-2697(91)90120-i. [DOI] [PubMed] [Google Scholar]
- Evers M. P., Zelle B., Bebelman J. P., van Beusechem V., Kraakman L., Hoffer M. J., Pronk J. C., Mager W. H., Planta R. J., Eriksson A. W. Nucleotide sequence comparison of five human pepsinogen A (PGA) genes: evolution of the PGA multigene family. Genomics. 1989 Apr;4(3):232–239. doi: 10.1016/0888-7543(89)90325-x. [DOI] [PubMed] [Google Scholar]
- Farrar G. J., Kenna P., Jordan S. A., Kumar-Singh R., Humphries M. M., Sharp E. M., Sheils D. M., Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Forsman K., Graff C., Nordström S., Johansson K., Westermark E., Lundgren E., Gustavson K. H., Wadelius C., Holmgren G. The gene for Best's macular dystrophy is located at 11q13 in a Swedish family. Clin Genet. 1992 Sep;42(3):156–159. doi: 10.1111/j.1399-0004.1992.tb03229.x. [DOI] [PubMed] [Google Scholar]
- Grimberg J., Nawoschik S., Belluscio L., McKee R., Turck A., Eisenberg A. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 1989 Oct 25;17(20):8390–8390. doi: 10.1093/nar/17.20.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Stewart P. W., Dilley W. G., Holt M. S., Steinbrueck T. D., Wells S. A., Jr, Donis-Keller H. A minisatellite and a microsatellite polymorphism within 1.5 kb at the human muscle glycogen phosphorylase (PYGM) locus can be amplified by PCR and have combined informativeness of PIC 0.95. Genomics. 1992 May;13(1):7–15. doi: 10.1016/0888-7543(92)90194-w. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Hahn L. B., Mukai S., Travis G. H., Berson E. L., Dryja T. P. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):480–483. doi: 10.1038/354480a0. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Sandberg M. A., Berson E. L., Dryja T. P. A null mutation in the human peripherin/RDS gene in a family with autosomal dominant retinitis punctata albescens. Nat Genet. 1993 Mar;3(3):208–212. doi: 10.1038/ng0393-208. [DOI] [PubMed] [Google Scholar]
- Kimberling W. J., Möller C. G., Davenport S., Priluck I. A., Beighton P. H., Greenberg J., Reardon W., Weston M. D., Kenyon J. B., Grunkemeyer J. A. Linkage of Usher syndrome type I gene (USH1B) to the long arm of chromosome 11. Genomics. 1992 Dec;14(4):988–994. doi: 10.1016/s0888-7543(05)80121-1. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Leibowitz H. M., Krueger D. E., Maunder L. R., Milton R. C., Kini M. M., Kahn H. A., Nickerson R. J., Pool J., Colton T. L., Ganley J. P. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980 May-Jun;24(Suppl):335–610. [PubMed] [Google Scholar]
- Li Y., Müller B., Fuhrmann C., van Nouhuys C. E., Laqua H., Humphries P., Schwinger E., Gal A. The autosomal dominant familial exudative vitreoretinopathy locus maps on 11q and is closely linked to D11S533. Am J Hum Genet. 1992 Oct;51(4):749–754. [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. E., Drack A. V., Vandenburgh K., Kimura A. E., Sheffield V. C., Stone E. M. A 2 base pair deletion in the RDS gene associated with butterfly-shaped pigment dystrophy of the fovea. Hum Mol Genet. 1993 May;2(5):601–603. doi: 10.1093/hmg/2.5.601. [DOI] [PubMed] [Google Scholar]
- Nichols B. E., Sheffield V. C., Vandenburgh K., Drack A. V., Kimura A. E., Stone E. M. Butterfly-shaped pigment dystrophy of the fovea caused by a point mutation in codon 167 of the RDS gene. Nat Genet. 1993 Mar;3(3):202–207. doi: 10.1038/ng0393-202. [DOI] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Lee E. C., Kimberling W. J., Daiger S. P., Pelias M. Z., Keats B. J., Jay M., Bird A., Reardon W., Guest M. Localization of two genes for Usher syndrome type I to chromosome 11. Genomics. 1992 Dec;14(4):995–1002. doi: 10.1016/s0888-7543(05)80122-3. [DOI] [PubMed] [Google Scholar]
- Stone E. M., Kimura A. E., Folk J. C., Bennett S. R., Nichols B. E., Streb L. M., Sheffield V. C. Genetic linkage of autosomal dominant neovascular inflammatory vitreoretinopathy to chromosome 11q13. Hum Mol Genet. 1992 Dec;1(9):685–689. doi: 10.1093/hmg/1.9.685. [DOI] [PubMed] [Google Scholar]
- Stone E. M., Nichols B. E., Streb L. M., Kimura A. E., Sheffield V. C. Genetic linkage of vitelliform macular degeneration (Best's disease) to chromosome 11q13. Nat Genet. 1992 Jul;1(4):246–250. doi: 10.1038/ng0792-246. [DOI] [PubMed] [Google Scholar]
- Wells J., Wroblewski J., Keen J., Inglehearn C., Jubb C., Eckstein A., Jay M., Arden G., Bhattacharya S., Fitzke F. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat Genet. 1993 Mar;3(3):213–218. doi: 10.1038/ng0393-213. [DOI] [PubMed] [Google Scholar]



