Abstract
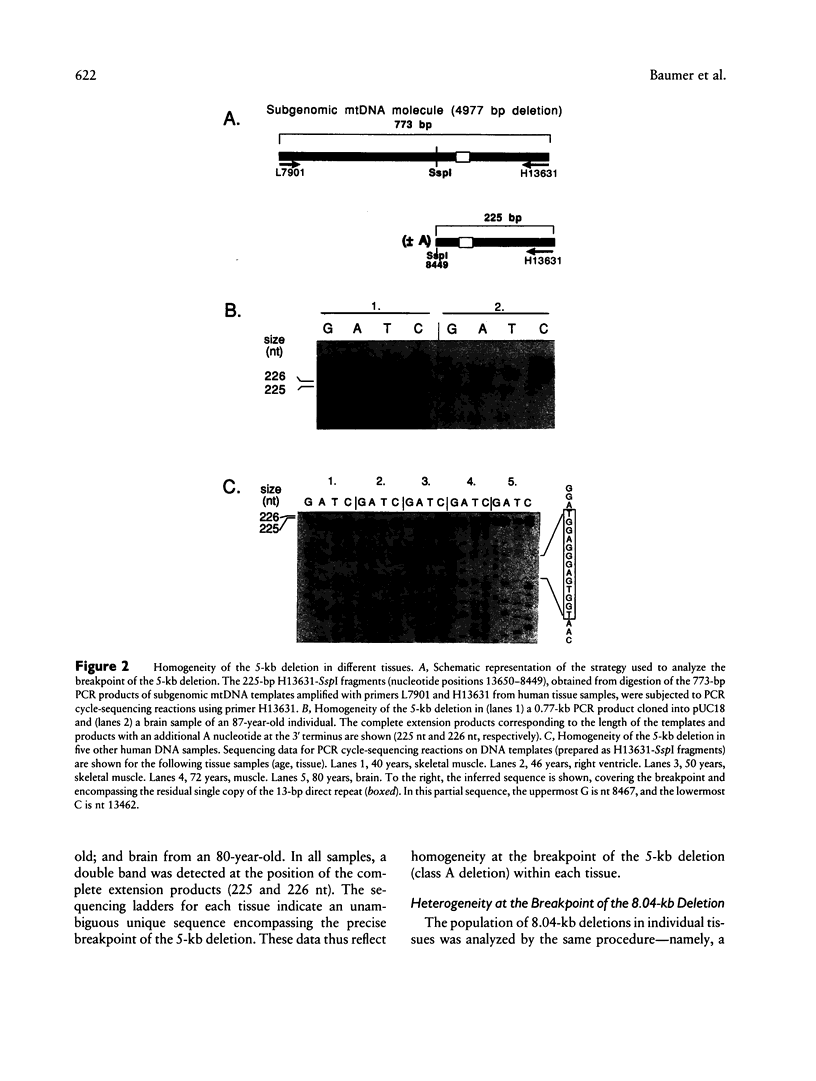
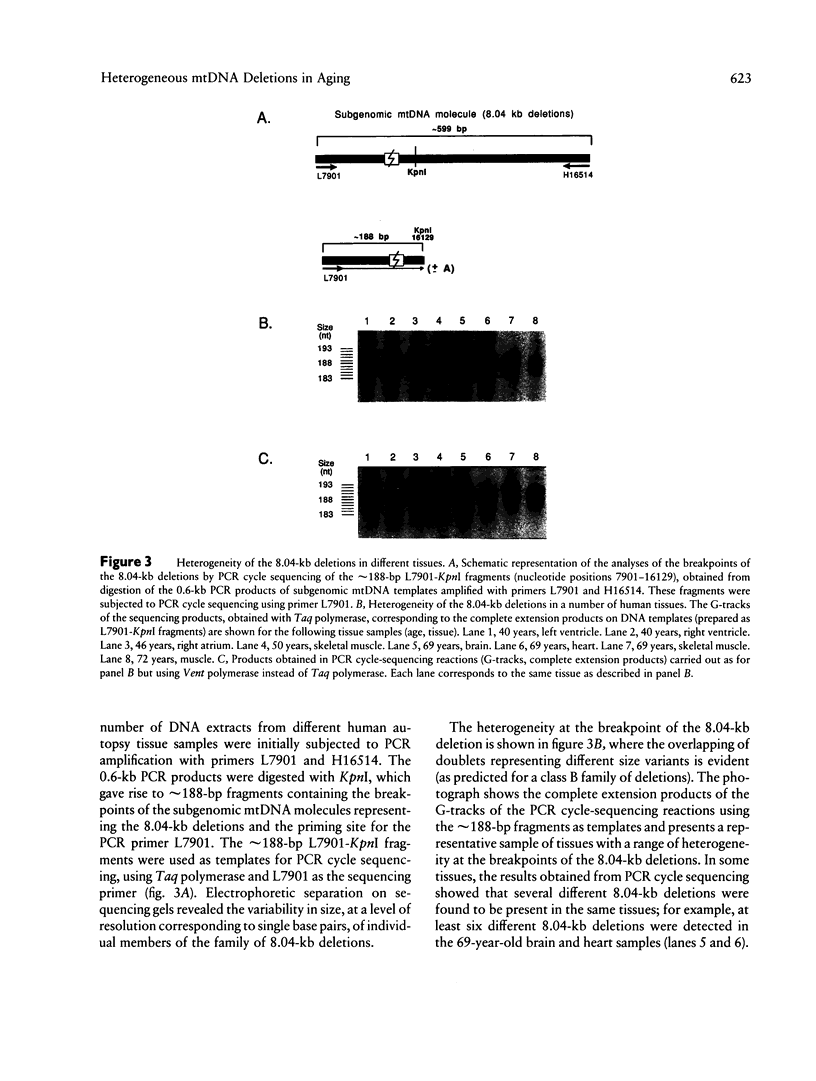
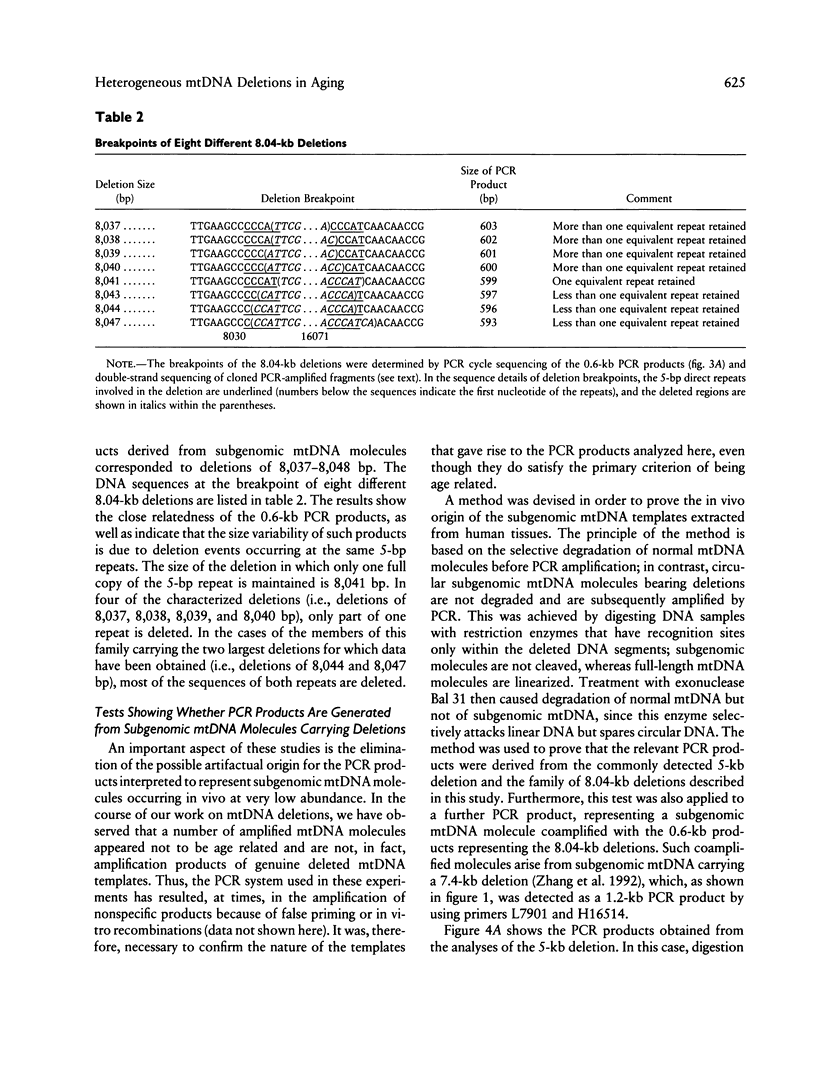
Deletions in mtDNA accumulate during the human aging process, arising from either intramolecular illegitimate recombination or strand slippage during replication, which results in subgenomic mtDNA molecules. We identify here two classes of mtDNA deletions--class A deletions, which are homogeneous at the breakpoints, with all subgenomic molecules therefore being identical in size, and class B deletions, which arise from a less stringent process that gives rise to heterogeneity at the breakpoints, with the subgenomic molecules being of slightly different sizes. A novel approach is described that offers a global overview of the populations of different deletions in individual tissues. It is based on PCR cycle-sequencing reactions that are carried out directly on mtDNA segments, amplified by PCR from total cellular DNA. The results show a clear size homogeneity of the subgenomic mtDNA molecules representative of class A, which carry a commonly detected 4,977-bp deletion occurring at a pair of 13-bp directly repeated sequences. In this case, precisely one copy of the repeat is retained in the subgenomic molecules. We then describe a class B situation comprising a family of at least nine closely related 8.04-kb deletions involving the same pair of 5-bp direct repeats. In this situation, the breakpoints differ at the base-pair level (8,037-8,048-bp deletions); the subgenomic molecules retain > 1 copy, 1 copy, or < 1 copy of the 5-bp repeat. In different tissues from either the same individual or among different individuals, there is a widely variable occurrence of particular deletions in the subgenomic mtDNA population within this class B set. Class B deletions offer a new approach for studying the accumulation of mtDNA deletions, thereby providing insight into the independent somatic origin of mutated mtDNA molecules, both in aging and in mitochondrial diseases. We also report a convenient method for ascertaining whether a given PCR product results from the amplification of a subgenomic mtDNA template, on the basis of the selective degradation of full-length mtDNA molecules prior to PCR.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Clark J. M. Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 1988 Oct 25;16(20):9677–9686. doi: 10.1093/nar/16.20.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortopassi G. A., Shibata D., Soong N. W., Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7370–7374. doi: 10.1073/pnas.89.16.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degoul F., Nelson I., Amselem S., Romero N., Obermaier-Kusser B., Ponsot G., Marsac C., Lestienne P. Different mechanisms inferred from sequences of human mitochondrial DNA deletions in ocular myopathies. Nucleic Acids Res. 1991 Feb 11;19(3):493–496. doi: 10.1093/nar/19.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Ogawa T., Kondo T., Mochizuki M., Tanaka M., Sugiyama S., Ito T., Satake T., Ozawa T. Cardiomyopathy with mitochondrial DNA mutations. Am Heart J. 1991 Sep;122(3 Pt 1):866–869. doi: 10.1016/0002-8703(91)90542-p. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Clayton D. A. Length heterogeneity of a conserved displacement-loop sequence in human mitochondrial DNA. Nucleic Acids Res. 1985 Nov 25;13(22):8093–8104. doi: 10.1093/nar/13.22.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I. J., Cooper J. M., Morgan-Hughes J. A., Harding A. E. Deletions of muscle mitochondrial DNA. Lancet. 1988 Jun 25;1(8600):1462–1462. doi: 10.1016/s0140-6736(88)92273-8. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Ikebe S., Tanaka M., Ohno K., Sato W., Hattori K., Kondo T., Mizuno Y., Ozawa T. Increase of deleted mitochondrial DNA in the striatum in Parkinson's disease and senescence. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1044–1048. doi: 10.1016/0006-291x(90)90497-b. [DOI] [PubMed] [Google Scholar]
- Larsson N. G., Eiken H. G., Boman H., Holme E., Oldfors A., Tulinius M. H. Lack of transmission of deleted mtDNA from a woman with Kearns-Sayre syndrome to her child. Am J Hum Genet. 1992 Feb;50(2):360–363. [PMC free article] [PubMed] [Google Scholar]
- Lestienne P., Ponsot G. Kearns-Sayre syndrome with muscle mitochondrial DNA deletion. Lancet. 1988 Apr 16;1(8590):885–885. doi: 10.1016/s0140-6736(88)91632-7. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Baumer A., Maxwell R. J., Preston H., Zhang C. F., Marzuki S. Mitochondrial gene mutation: the ageing process and degenerative diseases. Biochem Int. 1990 Dec;22(6):1067–1076. [PubMed] [Google Scholar]
- Linnane A. W., Marzuki S., Ozawa T., Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989 Mar 25;1(8639):642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Zhang C., Baumer A., Nagley P. Mitochondrial DNA mutation and the ageing process: bioenergy and pharmacological intervention. Mutat Res. 1992 Sep;275(3-6):195–208. doi: 10.1016/0921-8734(92)90023-i. [DOI] [PubMed] [Google Scholar]
- Münscher C., Rieger T., Müller-Höcker J., Kadenbach B. The point mutation of mitochondrial DNA characteristic for MERRF disease is found also in healthy people of different ages. FEBS Lett. 1993 Feb 8;317(1-2):27–30. doi: 10.1016/0014-5793(93)81484-h. [DOI] [PubMed] [Google Scholar]
- Obermaier-Kusser B., Müller-Höcker J., Nelson I., Lestienne P., Enter C., Riedele T., Gerbitz K. D. Different copy numbers of apparently identically deleted mitochondrial DNA in tissues from a patient with Kearns-Sayre syndrome detected by PCR. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1007–1015. doi: 10.1016/0006-291x(90)91994-4. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Sugiyama S., Hattori K., Ito T., Ohno K., Takahashi A., Sato W., Takada G., Mayumi B. Multiple mitochondrial DNA deletions exist in cardiomyocytes of patients with hypertrophic or dilated cardiomyopathy. Biochem Biophys Res Commun. 1990 Jul 31;170(2):830–836. doi: 10.1016/0006-291x(90)92166-w. [DOI] [PubMed] [Google Scholar]
- Poulton J., Deadman M. E., Bindoff L., Morten K., Land J., Brown G. Families of mtDNA re-arrangements can be detected in patients with mtDNA deletions: duplications may be a transient intermediate form. Hum Mol Genet. 1993 Jan;2(1):23–30. doi: 10.1093/hmg/2.1.23. [DOI] [PubMed] [Google Scholar]
- Rotig A., Colonna M., Bonnefont J. P., Blanche S., Fischer A., Saudubray J. M., Munnich A. Mitochondrial DNA deletion in Pearson's marrow/pancreas syndrome. Lancet. 1989 Apr 22;1(8643):902–903. doi: 10.1016/s0140-6736(89)92897-3. [DOI] [PubMed] [Google Scholar]
- Rötig A., Colonna M., Blanche S., Fischer A., Le Deist F., Frezal J., Saudubray J. M., Munnich A. Deletion of blood mitochondrial DNA in pancytopenia. Lancet. 1988 Sep 3;2(8610):567–568. doi: 10.1016/s0140-6736(88)92687-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Voljavec A. S., Soueidan S. A., Costigan D. A., Wallace D. C. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounce I., Byrne E., Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989 Mar 25;1(8639):637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wei Y. H. Mitochondrial DNA alterations as ageing-associated molecular events. Mutat Res. 1992 Sep;275(3-6):145–155. doi: 10.1016/0921-8734(92)90019-l. [DOI] [PubMed] [Google Scholar]
- Yen T. C., Chen Y. S., King K. L., Yeh S. H., Wei Y. H. Liver mitochondrial respiratory functions decline with age. Biochem Biophys Res Commun. 1989 Dec 29;165(3):944–1003. doi: 10.1016/0006-291x(89)92701-0. [DOI] [PubMed] [Google Scholar]
- Yen T. C., Pang C. Y., Hsieh R. H., Su C. H., King K. L., Wei Y. H. Age-dependent 6kb deletion in human liver mitochondrial DNA. Biochem Int. 1992 Mar;26(3):457–468. [PubMed] [Google Scholar]
- Yen T. C., Su J. H., King K. L., Wei Y. H. Ageing-associated 5 kb deletion in human liver mitochondrial DNA. Biochem Biophys Res Commun. 1991 Jul 15;178(1):124–131. doi: 10.1016/0006-291x(91)91788-e. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Servidei S., Gellera C., Bertini E., DiMauro S., DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989 May 25;339(6222):309–311. doi: 10.1038/339309a0. [DOI] [PubMed] [Google Scholar]
- Zhang C., Baumer A., Maxwell R. J., Linnane A. W., Nagley P. Multiple mitochondrial DNA deletions in an elderly human individual. FEBS Lett. 1992 Feb 3;297(1-2):34–38. doi: 10.1016/0014-5793(92)80321-7. [DOI] [PubMed] [Google Scholar]
- Zhang C., Linnane A. W., Nagley P. Occurrence of a particular base substitution (3243 A to G) in mitochondrial DNA of tissues of ageing humans. Biochem Biophys Res Commun. 1993 Sep 15;195(2):1104–1110. doi: 10.1006/bbrc.1993.2158. [DOI] [PubMed] [Google Scholar]