Abstract
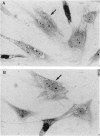
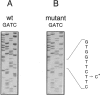
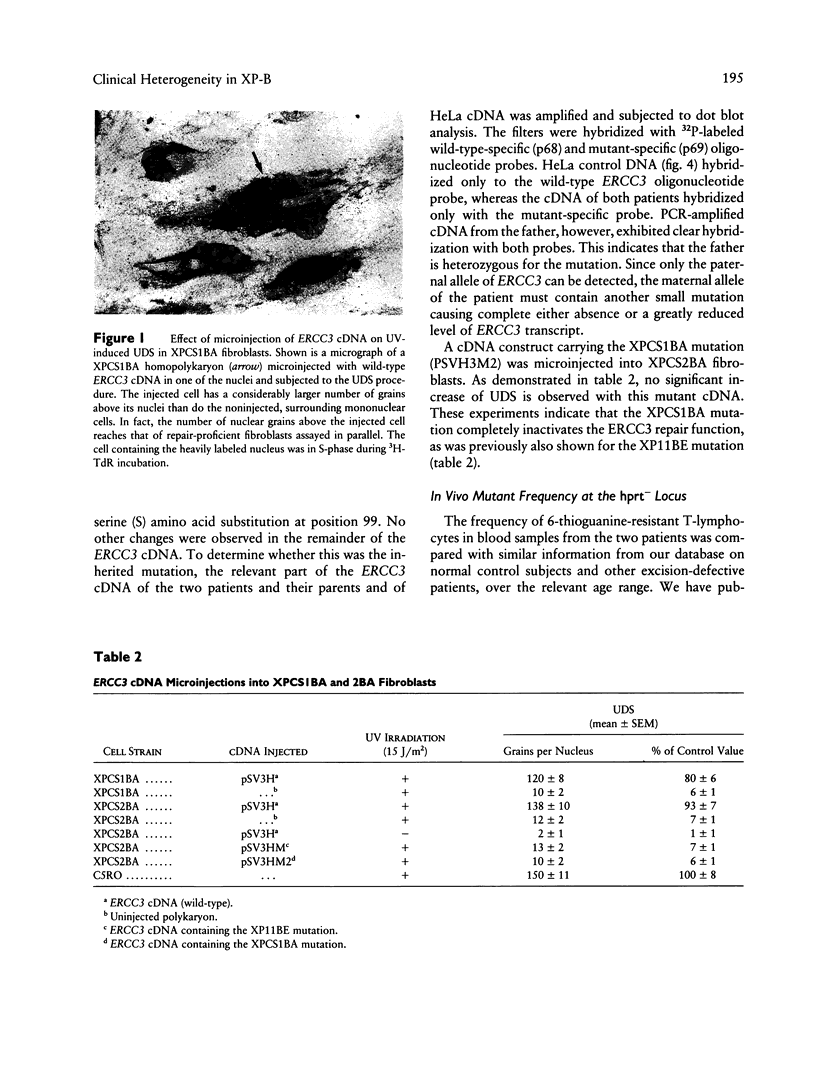
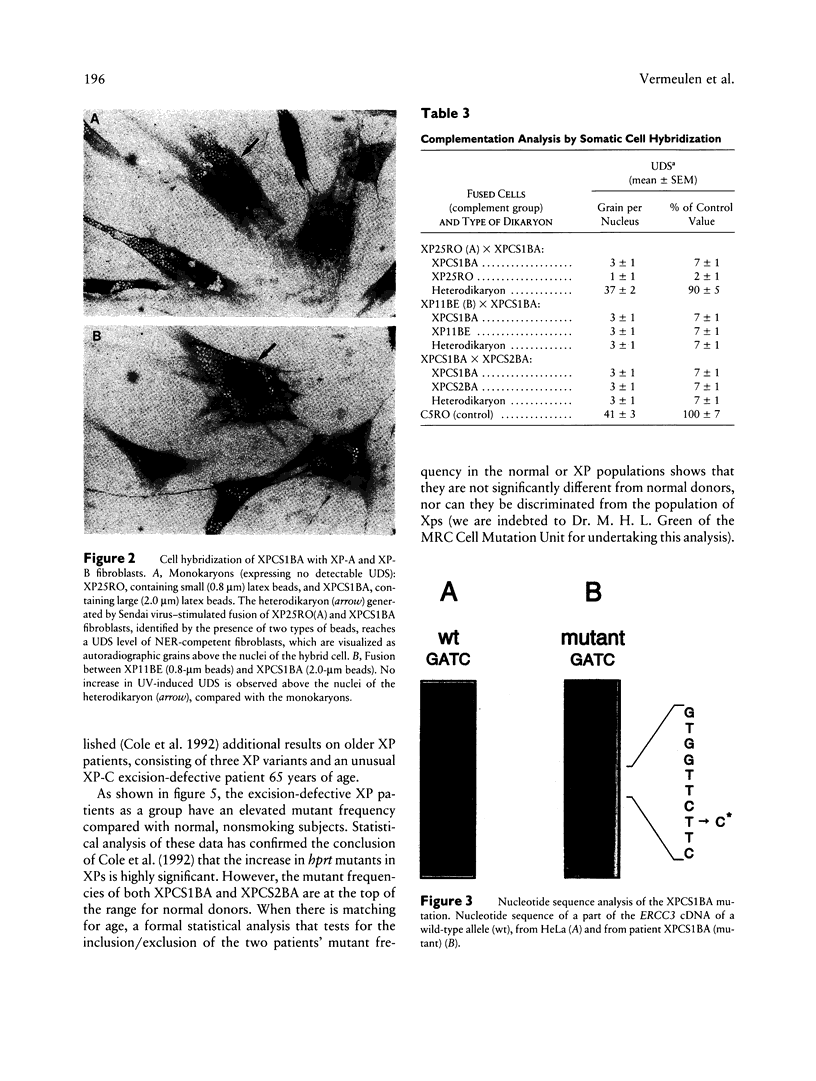
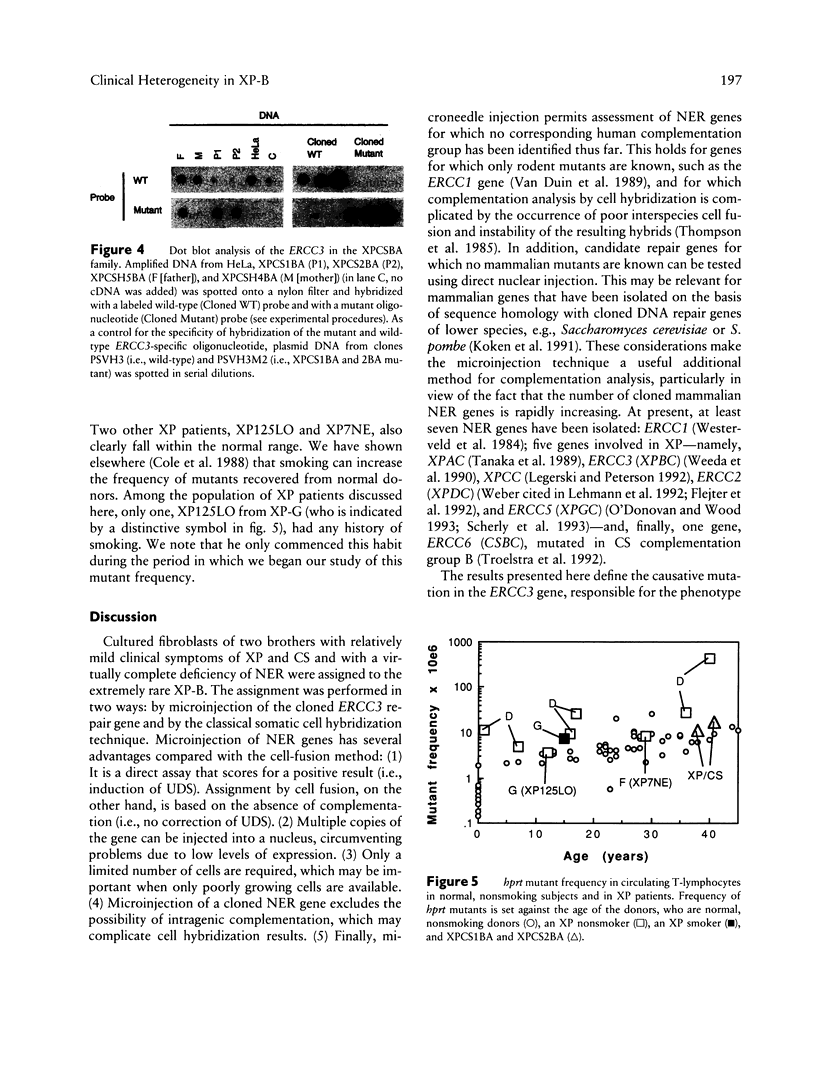
The human DNA excision repair gene ERCC3 specifically corrects the nucleotide excision repair (NER) defect of xeroderma pigmentosum (XP) complementation group B. In addition to its function in NER, the ERCC3 DNA helicase was recently identified as one of the components of the human BTF2/TFIIH transcription factor complex, which is required for initiation of transcription of class II genes. To date, a single patient (XP11BE) has been assigned to this XP group B (XP-B), with ther remarkable conjunction of two autosomal recessive DNA repair deficiency disorders: XP and Cockayne syndrome (CS). The intriguing involvement of the ERCC3 protein in the vital process of transcription may provide an explanation for the rarity, severity, and wide spectrum of clinical features in this complementation group. Here we report the identification of two new XP-B patients: XPCS1BA and XPCS2BA (siblings), by microneedle injection of the cloned ERCC3 repair gene as well as by cell hybridization. Molecular analysis of the ERCC3 gene in both patients revealed a single base substitution causing a missense mutation in a region that is completely conserved in yeast, Drosophila, mouse, and human ERCC3. As in patient XP11BE, the expression of only one allele (paternal) is detected. The mutation causes a virtually complete inactivation of the NER function of the protein. Despite this severe NER defect, both patients display a late onset of neurologic impairment, mild cutaneous symptoms, and a striking absence of skin tumors even at an age of > 40 years. Analysis of the frequency of hprt- mutant T-lymphocytes in blood samples suggests a relatively low in vivo mutation frequency in these patients. Factors in addition to NER deficiency may be required for the development of cutaneous tumors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlett C. F., Harcourt S. A., Cole J., Green M. H., Anstey A. V. A comparison of the response of unstimulated and stimulated T-lymphocytes and fibroblasts from normal, xeroderma pigmentosum and trichothiodystrophy donors to the lethal action of UV-C. Mutat Res. 1992 Mar;273(2):127–135. doi: 10.1016/0921-8777(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bohr V. A. Gene specific DNA repair. Carcinogenesis. 1991 Nov;12(11):1983–1992. doi: 10.1093/carcin/12.11.1983. [DOI] [PubMed] [Google Scholar]
- Bootsma D., Hoeijmakers J. H. DNA repair. Engagement with transcription. Nature. 1993 May 13;363(6425):114–115. doi: 10.1038/363114a0. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Brown G. M. Mutagenic DNA repair in Escherichia coli. XXI. A stable SOS-inducing signal persisting after excision repair of ultraviolet damage. Mutat Res. 1992 Nov 16;270(2):135–144. doi: 10.1016/0027-5107(92)90124-k. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cole J., Arlett C. F., Norris P. G., Stephens G., Waugh A. P., Beare D. M., Green M. H. Elevated hprt mutant frequency in circulating T-lymphocytes of xeroderma pigmentosum patients. Mutat Res. 1992 Mar;273(2):171–178. doi: 10.1016/0921-8777(92)90078-h. [DOI] [PubMed] [Google Scholar]
- Cole J., Green M. H., James S. E., Henderson L., Cole H. A further assessment of factors influencing measurements of thioguanine-resistant mutant frequency in circulating T-lymphocytes. Mutat Res. 1988 Mar;204(3):493–507. doi: 10.1016/0165-1218(88)90044-4. [DOI] [PubMed] [Google Scholar]
- Flejter W. L., McDaniel L. D., Johns D., Friedberg E. C., Schultz R. A. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: involvement of the human ERCC2 DNA repair gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):261–265. doi: 10.1073/pnas.89.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas K. D., Donahue T. F. SSL2, a suppressor of a stem-loop mutation in the HIS4 leader encodes the yeast homolog of human ERCC-3. Cell. 1992 Jun 12;69(6):1031–1042. doi: 10.1016/0092-8674(92)90621-i. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. Heterogeneity of DNA repair at the gene level. Mutat Res. 1991 Apr;247(2):203–211. doi: 10.1016/0027-5107(91)90016-h. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair. II: From yeast to mammals. Trends Genet. 1993 Jun;9(6):211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- Koken M. H., Reynolds P., Jaspers-Dekker I., Prakash L., Prakash S., Bootsma D., Hoeijmakers J. H. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8865–8869. doi: 10.1073/pnas.88.20.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R., Peterson C. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature. 1992 Sep 3;359(6390):70–73. doi: 10.1038/359070a0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Bridges B. A. Sunlight-induced cancer: some new aspects and implications of the xeroderma pigmentosum model. Br J Dermatol. 1990 Apr;122 (Suppl 35):115–119. doi: 10.1111/j.1365-2133.1990.tb16136.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Hoeijmakers J. H., van Zeeland A. A., Backendorf C. M., Bridges B. A., Collins A., Fuchs R. P., Margison G. P., Montesano R., Moustacchi E. Workshop on DNA repair. Mutat Res. 1992 Jan;273(1):1–28. doi: 10.1016/0921-8777(92)90046-6. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R. Three complementation groups in Cockayne syndrome. Mutat Res. 1982 Dec;106(2):347–356. doi: 10.1016/0027-5107(82)90115-4. [DOI] [PubMed] [Google Scholar]
- Mariani E., Facchini A., Honorati M. C., Lalli E., Berardesca E., Ghetti P., Marinoni S., Nuzzo F., Astaldi Ricotti G. C., Stefanini M. Immune defects in families and patients with xeroderma pigmentosum and trichothiodystrophy. Clin Exp Immunol. 1992 Jun;88(3):376–382. doi: 10.1111/j.1365-2249.1992.tb06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison W. L., Bucana C., Hashem N., Kripke M. L., Cleaver J. E., German J. L. Impaired immune function in patients with xeroderma pigmentosum. Cancer Res. 1985 Aug;45(8):3929–3931. [PubMed] [Google Scholar]
- Mounkes L. C., Jones R. S., Liang B. C., Gelbart W., Fuller M. T. A Drosophila model for xeroderma pigmentosum and Cockayne's syndrome: haywire encodes the fly homolog of ERCC3, a human excision repair gene. Cell. 1992 Dec 11;71(6):925–937. doi: 10.1016/0092-8674(92)90389-t. [DOI] [PubMed] [Google Scholar]
- Nance M. A., Berry S. A. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992 Jan 1;42(1):68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- O'Donovan A., Wood R. D. Identical defects in DNA repair in xeroderma pigmentosum group G and rodent ERCC group 5. Nature. 1993 May 13;363(6425):185–188. doi: 10.1038/363185a0. [DOI] [PubMed] [Google Scholar]
- Park E., Guzder S. N., Koken M. H., Jaspers-Dekker I., Weeda G., Hoeijmakers J. H., Prakash S., Prakash L. RAD25 (SSL2), the yeast homolog of the human xeroderma pigmentosum group B DNA repair gene, is essential for viability. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11416–11420. doi: 10.1073/pnas.89.23.11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. H., Brumback R. A., Mendiones M., Barrett S. F., Carl J. R., Cho S., Denckla M. B., Ganges M. B., Gerber L. H., Guthrie R. A. Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain. 1991 Jun;114(Pt 3):1335–1361. doi: 10.1093/brain/114.3.1335. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Scherly D., Nouspikel T., Corlet J., Ucla C., Bairoch A., Clarkson S. G. Complementation of the DNA repair defect in xeroderma pigmentosum group G cells by a human cDNA related to yeast RAD2. Nature. 1993 May 13;363(6425):182–185. doi: 10.1038/363182a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Satokata I., Ogita Z., Uchida T., Okada Y. Molecular cloning of a mouse DNA repair gene that complements the defect of group-A xeroderma pigmentosum. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5512–5516. doi: 10.1073/pnas.86.14.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mooney C. L., Brookman K. W. Genetic complementation between UV-sensitive CHO mutants and xeroderma pigmentosum fibroblasts. Mutat Res. 1985 Jun-Jul;150(1-2):423–429. doi: 10.1016/0027-5107(85)90139-3. [DOI] [PubMed] [Google Scholar]
- Troelstra C., van Gool A., de Wit J., Vermeulen W., Bootsma D., Hoeijmakers J. H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell. 1992 Dec 11;71(6):939–953. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- Venema J., Mullenders L. H., Natarajan A. T., van Zeeland A. A., Mayne L. V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Natarajan A. T., van Zeeland A. A., Mullenders L. H. The residual repair capacity of xeroderma pigmentosum complementation group C fibroblasts is highly specific for transcriptionally active DNA. Nucleic Acids Res. 1990 Feb 11;18(3):443–448. doi: 10.1093/nar/18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen W., Jaeken J., Jaspers N. G., Bootsma D., Hoeijmakers J. H. Xeroderma pigmentosum complementation group G associated with Cockayne syndrome. Am J Hum Genet. 1993 Jul;53(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- Vermeulen W., Osseweijer P., de Jonge A. J., Hoeijmakers J. H. Transient correction of excision repair defects in fibroblasts of 9 xeroderma pigmentosum complementation groups by microinjection of crude human cell extracts. Mutat Res. 1986 May;165(3):199–206. doi: 10.1016/0167-8817(86)90055-6. [DOI] [PubMed] [Google Scholar]
- Vermeulen W., Stefanini M., Giliani S., Hoeijmakers J. H., Bootsma D. Xeroderma pigmentosum complementation group H falls into complementation group D. Mutat Res. 1991 Sep;255(2):201–208. doi: 10.1016/0921-8777(91)90054-s. [DOI] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- van Duin M., Vredeveldt G., Mayne L. V., Odijk H., Vermeulen W., Klein B., Weeda G., Hoeijmakers J. H., Bootsma D., Westerveld A. The cloned human DNA excision repair gene ERCC-1 fails to correct xeroderma pigmentosum complementation groups A through I. Mutat Res. 1989 Mar;217(2):83–92. doi: 10.1016/0921-8777(89)90059-1. [DOI] [PubMed] [Google Scholar]