Abstract
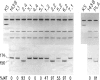
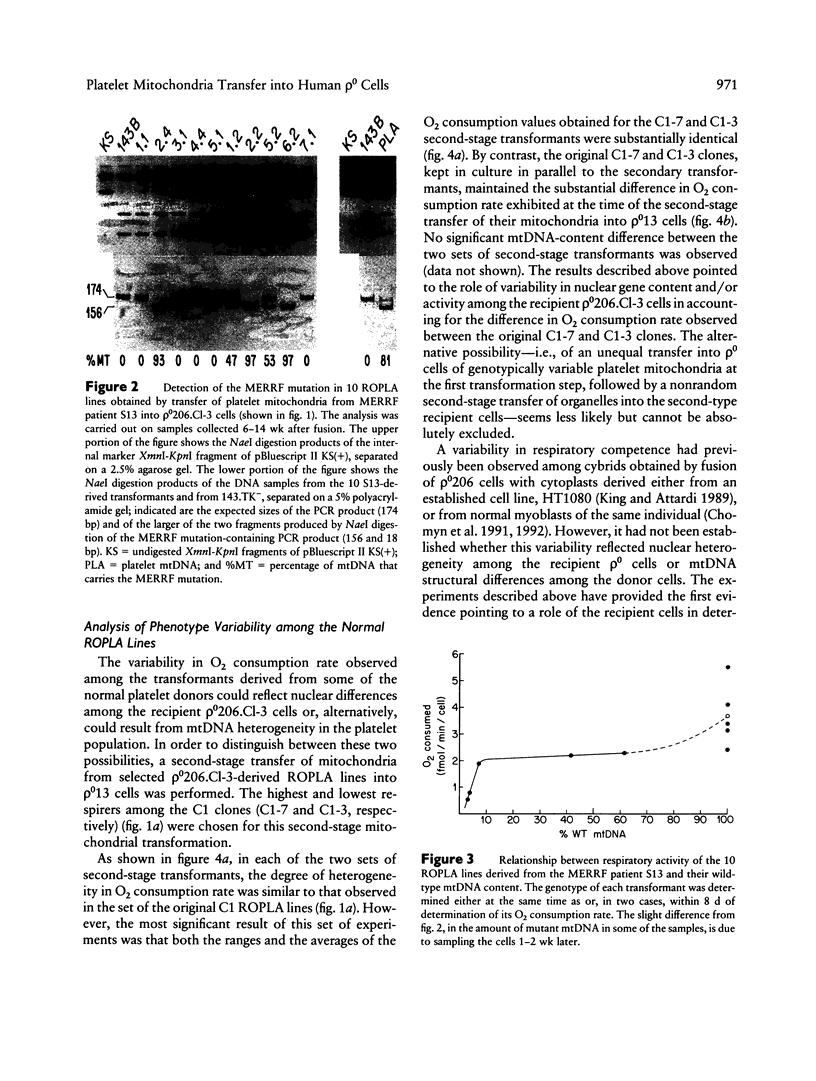
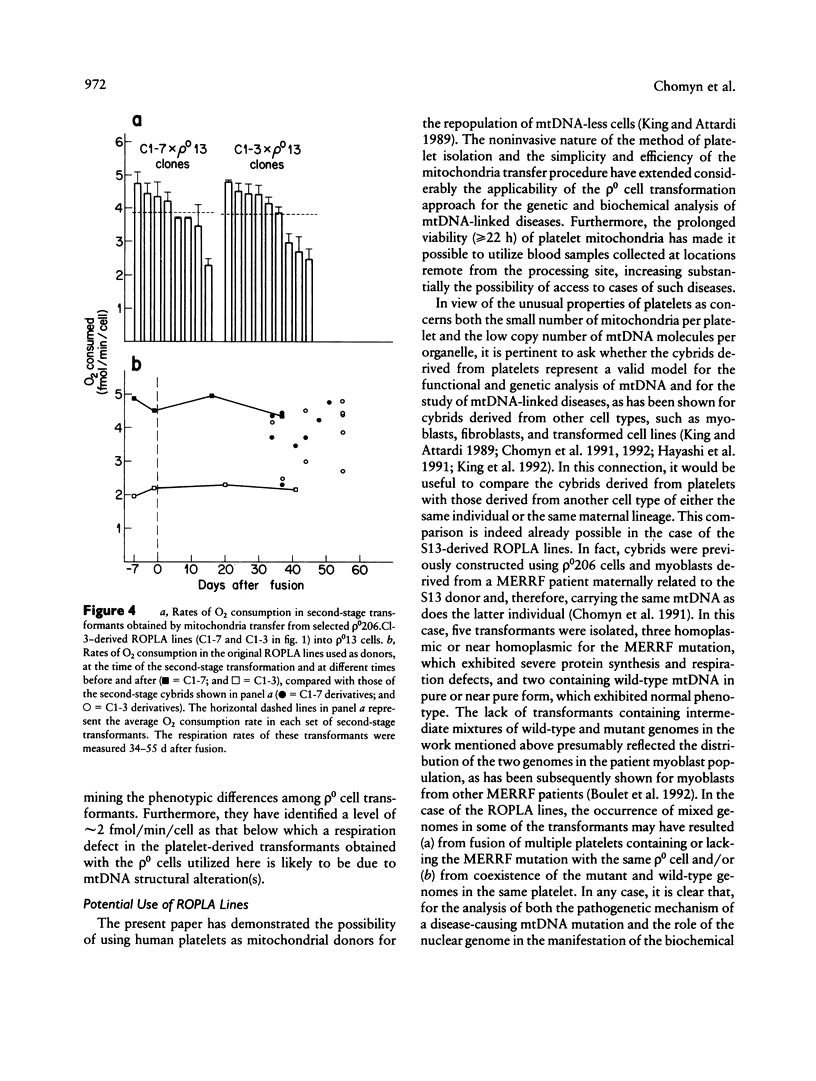
In the present work, we demonstrate the possibility of using human blood platelets as mitochondrial donors for the repopulation of mtDNA-less (rho 0) cells. The noninvasive nature of platelet isolation, combined with the prolonged viability of platelet mitochondria and the simplicity and efficiency of the mitochondria-transfer procedure, has substantially increased the applicability of the rho 0 cell transformation approach for mitochondrial genetic analysis and for the study of mtDNA-linked diseases. This approach has been applied to platelets from several normal human individuals and one individual affected by the myoclonic-epilepsy-and-ragged-red-fibers (MERRF) encephalomyopathy. A certain variability in respiratory capacity was observed among the platelet-derived rho 0 cell transformants from a given normal subject, and it was shown to be unrelated to their mtDNA content. The results of sequential transfer of mitochondria from selected transformants into a rho 0 cell line different from the first rho 0 acceptor strongly suggest that this variability reflected, at least in part, differences in nuclear gene content and/or activity among the original recipient cells. A much greater variability in respiratory capacity was observed among the transformants derived from the MERRF patient and was found to be related to the presence and amount of the mitochondrial tRNALys mutation associated with the MERRF syndrome. An analysis of the relationship between proportion of mtDNA carrying the MERRF mutation and degree of respiratory activity in various transformants derived from the MERRF patient revealed an unusual complementation behavior of the tRNALys mutation, possibly reflecting the distribution of mutant mtDNA among the platelet mitochondria.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams E. S., Stanton V. P., Jr Use of denaturing gradient gel electrophoresis to study conformational transitions in nucleic acids. Methods Enzymol. 1992;212:71–104. doi: 10.1016/0076-6879(92)12006-c. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Benecke R., Strümper P., Weiss H. Electron transfer complexes I and IV of platelets are abnormal in Parkinson's disease but normal in Parkinson-plus syndromes. Brain. 1993 Dec;116(Pt 6):1451–1463. doi: 10.1093/brain/116.6.1451. [DOI] [PubMed] [Google Scholar]
- Bindoff L. A., Birch-Machin M. A., Cartlidge N. E., Parker W. D., Jr, Turnbull D. M. Respiratory chain abnormalities in skeletal muscle from patients with Parkinson's disease. J Neurol Sci. 1991 Aug;104(2):203–208. doi: 10.1016/0022-510x(91)90311-t. [DOI] [PubMed] [Google Scholar]
- Boulet L., Karpati G., Shoubridge E. A. Distribution and threshold expression of the tRNA(Lys) mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1992 Dec;51(6):1187–1200. [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Martinuzzi A., Yoneda M., Daga A., Hurko O., Johns D., Lai S. T., Nonaka I., Angelini C., Attardi G. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez I. L., Gorski J. L., Campen T. J., Dorney D. J., Erickson J. M., Sylvester J. E., Schmickel R. D. Variation among human 28S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7666–7670. doi: 10.1073/pnas.82.22.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kikuchi A., Takemitsu M., Goto Y., Nonaka I. Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10614–10618. doi: 10.1073/pnas.88.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krige D., Carroll M. T., Cooper J. M., Marsden C. D., Schapira A. H. Platelet mitochondrial function in Parkinson's disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann Neurol. 1992 Dec;32(6):782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- Mann V. M., Cooper J. M., Krige D., Daniel S. E., Schapira A. H., Marsden C. D. Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson's disease. Brain. 1992 Apr;115(Pt 2):333–342. doi: 10.1093/brain/115.2.333. [DOI] [PubMed] [Google Scholar]
- Mason T. L., Poyton R. O., Wharton D. C., Schatz G. Cytochrome c oxidase from bakers' yeast. I. Isolation and properties. J Biol Chem. 1973 Feb 25;248(4):1346–1354. [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Sugiyama S., Ino H., Ohno K., Hattori K., Ohbayashi T., Ito T., Deguchi H., Kawamura K. Patients with idiopathic cardiomyopathy belong to the same mitochondrial DNA gene family of Parkinson's disease and mitochondrial encephalomyopathy. Biochem Biophys Res Commun. 1991 May 31;177(1):518–525. doi: 10.1016/0006-291x(91)92014-b. [DOI] [PubMed] [Google Scholar]
- Parker W. D., Jr, Boyson S. J., Parks J. K. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989 Dec;26(6):719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Parker W. D., Jr, Filley C. M., Parks J. K. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990 Aug;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Brown M. D., Torroni A., Lott M. T., Cabell M. F., Mirra S. S., Beal M. F., Yang C. C., Gearing M., Salvo R. Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics. 1993 Jul;17(1):171–184. doi: 10.1006/geno.1993.1299. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Shuster R. C., Rubenstein A. J., Wallace D. C. Mitochondrial DNA in anucleate human blood cells. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1360–1365. doi: 10.1016/s0006-291x(88)81291-9. [DOI] [PubMed] [Google Scholar]
- Storrie B., Attardi G. Expression of the mitochondrial genome in HeLa cells. 13. Effect of selective inhibition of cytoplasmic or mitochondrial protein synthesis on mitochondrial nucleic acid synthesis. J Mol Biol. 1972 Nov 14;71(2):177–199. doi: 10.1016/0022-2836(72)90345-2. [DOI] [PubMed] [Google Scholar]
- Tanno Y., Yoneda M., Nonaka I., Tanaka K., Miyatake T., Tsuji S. Quantitation of mitochondrial DNA carrying tRNALys mutation in MERRF patients. Biochem Biophys Res Commun. 1991 Sep 16;179(2):880–885. doi: 10.1016/0006-291x(91)91900-w. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Chomyn A., Martinuzzi A., Hurko O., Attardi G. Marked replicative advantage of human mtDNA carrying a point mutation that causes the MELAS encephalomyopathy. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11164–11168. doi: 10.1073/pnas.89.23.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M., Tanno Y., Horai S., Ozawa T., Miyatake T., Tsuji S. A common mitochondrial DNA mutation in the t-RNA(Lys) of patients with myoclonus epilepsy associated with ragged-red fibers. Biochem Int. 1990 Aug;21(5):789–796. [PubMed] [Google Scholar]
- Yoshino H., Nakagawa-Hattori Y., Kondo T., Mizuno Y. Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson's disease. J Neural Transm Park Dis Dement Sect. 1992;4(1):27–34. doi: 10.1007/BF02257619. [DOI] [PubMed] [Google Scholar]