Abstract
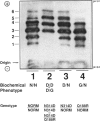
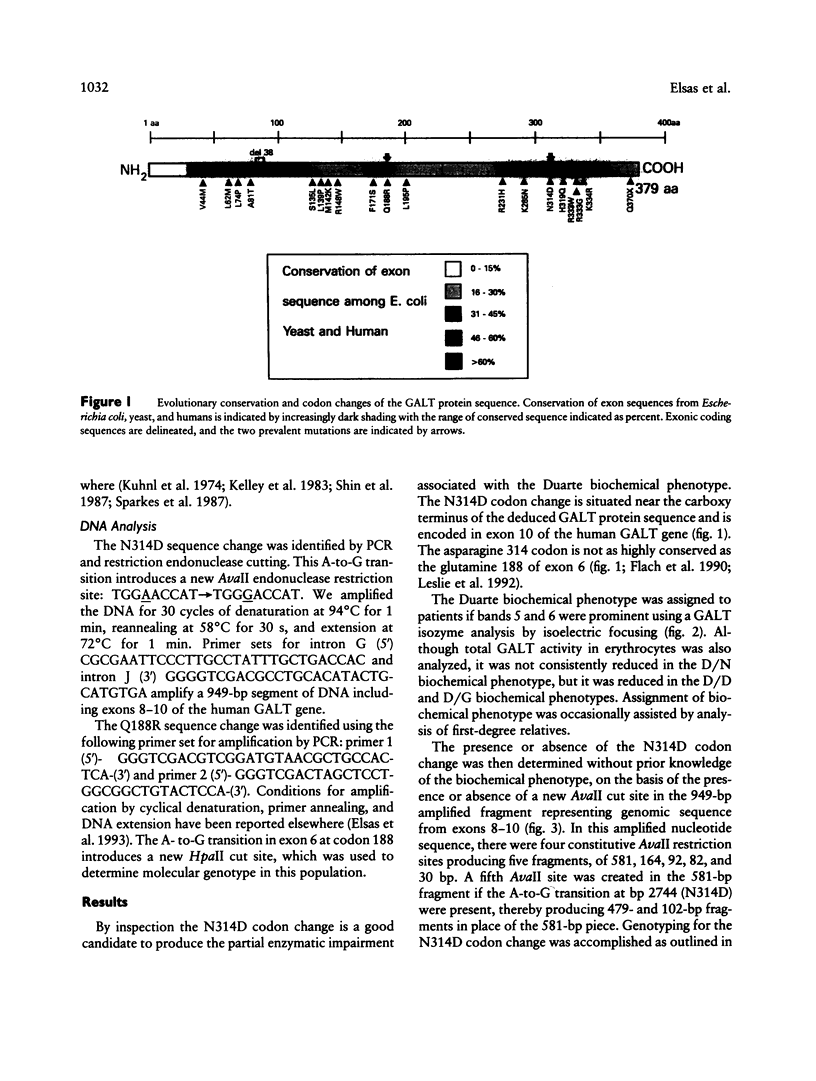
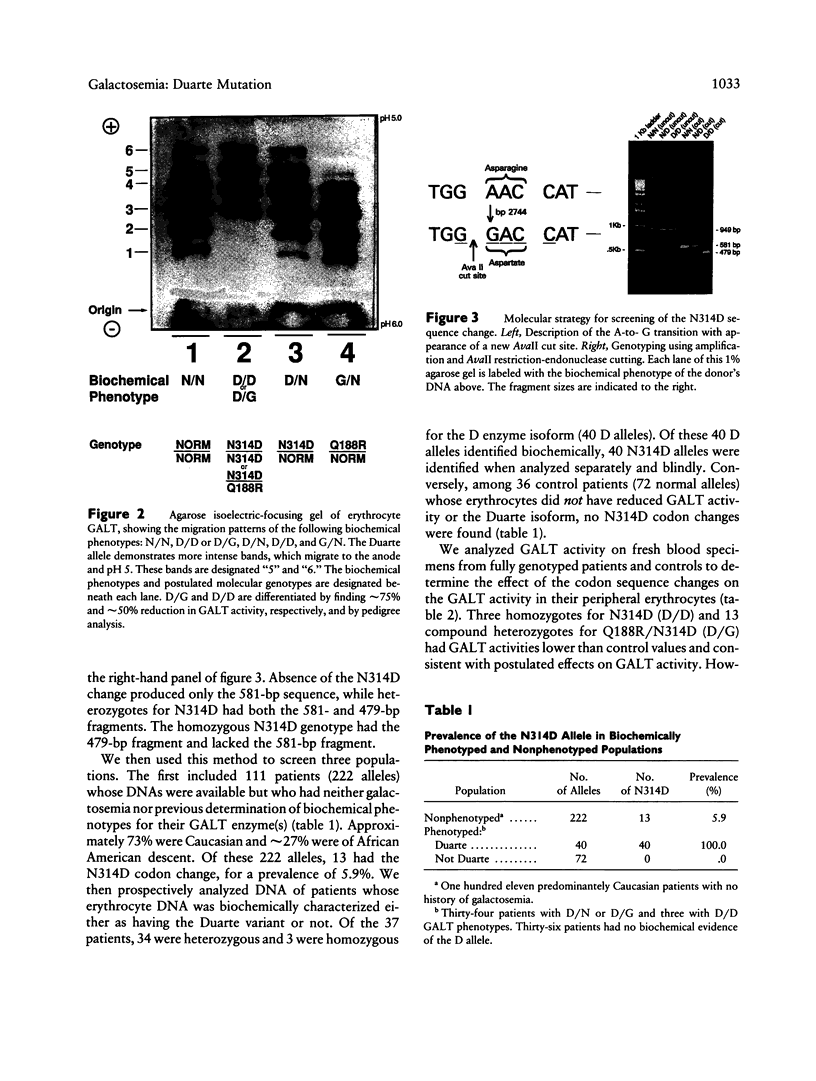
The human cDNA and gene for galactose-1-phosphate uridyl transferase (GALT) have been cloned and sequenced. A prevalent mutation (Q188R) is known to cause classic galactosemia (G/G). G/G galactosemia has an incidence of 1/38,886 in 1,396,766 Georgia live-born infants, but a more common variant of galactosemia, Duarte, has an unknown incidence. The proposed Duarte biochemical phenotypes of GALT are as follows: D/N, D/D, and D/G, which have approximately 75%, 50%, and 25% of normal GALT activity respectively. In addition, the D allele has isoforms of its enzyme that have more acidic pI than normal. Here we systematically determine (a) the prevalence of an A-to-G transition at base pair 2744 of exon 10 in the GALT gene, transition that produces a codon change converting asparagine to aspartic acid at position 314 (N314D), and (b) the association of this mutation with the Duarte biochemical phenotype. The 2744G nucleotide change adds an AvaII (SinI) cut site, which was identified in PCR-amplified DNA. In 111 biochemically unphenotyped controls with no history of galactosemia, 13 N314D alleles were identified (prevalence 5.9%). In a prospective study, 40 D alleles were biochemically phenotyped, and 40 N314D alleles were found. By contrast, in 36 individuals known not to have the Duarte biochemical phenotype, no N314D alleles were found. We conclude that the N314D mutation is a common allele that probably causes the Duarte GALT biochemical phenotype and occurs in a predominantly Caucasian, nongalactosemic population, with a prevalence of 5.9%.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. W., Williams V. P., Sparkes M. C., Sparkes R. S. Transferase-deficiency galactosemia: immunochemical studies of the Duarte and Los Angeles variants. Hum Genet. 1984;65(3):287–290. doi: 10.1007/BF00286519. [DOI] [PubMed] [Google Scholar]
- BEUTLER E., BALUDA M. C., STURGEON P., DAY R. A NEW GENETIC ABNORMALITY RESULTING IN GALACTOSE-1-PHOSPHATE URIDYLTRANSFERASE DEFICIENCY. Lancet. 1965 Feb 13;1(7381):353–354. doi: 10.1016/s0140-6736(65)91782-4. [DOI] [PubMed] [Google Scholar]
- Beutler E. Screening for galactosemia. Studies of the gene frequencies for galactosemia and the Duarte variant. Isr J Med Sci. 1973 Sep-Oct;9(9):1323–1329. [PubMed] [Google Scholar]
- DONNELL G. N., COLLADO M., KOCH R. Growth and development of children with galactosemia. J Pediatr. 1961 Jun;58:836–844. doi: 10.1016/s0022-3476(61)80139-x. [DOI] [PubMed] [Google Scholar]
- Flach J. E., Reichardt J. K., Elsas L. J., 2nd Sequence of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1990 Aug;7(4):365–369. [PubMed] [Google Scholar]
- Fox J. G. Experience of the Manitoba Perinatal Screening Program, 1965-85. CMAJ. 1987 Nov 15;137(10):883–888. [PMC free article] [PubMed] [Google Scholar]
- Frey P. A., Wong L. J., Sheu K. F., Yang S. L. Galactose-1-phosphate uridylyltransferase: detection, isolation, and characterization of the uridylyl enzyme. Methods Enzymol. 1982;87:20–36. doi: 10.1016/s0076-6879(82)87004-3. [DOI] [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Jinks-Robertson S. A yeast expression system for human galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):398–402. doi: 10.1073/pnas.90.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg C. R., Dilling L. A., Thompson R., Ford J. D., Seargeant L. E., Haworth J. C. Newborn screening for galactosemia: a new method used in Manitoba. Pediatrics. 1989 Aug;84(2):331–335. [PubMed] [Google Scholar]
- Ibarra B., Vaca G., Sánchez-Corona J., Hernández A., Ramirez M. L., Cantú J. M. Los Angeles variant of galactose-1-phosphate uridyltransferase (EC 2.7.7.12) in a Mexican family. Hum Genet. 1979 Apr 17;48(1):121–124. doi: 10.1007/BF00273284. [DOI] [PubMed] [Google Scholar]
- Kaufman F. R., Kogut M. D., Donnell G. N., Goebelsmann U., March C., Koch R. Hypergonadotropic hypogonadism in female patients with galactosemia. N Engl J Med. 1981 Apr 23;304(17):994–998. doi: 10.1056/NEJM198104233041702. [DOI] [PubMed] [Google Scholar]
- Kelley R. I., Harris H., Mellman W. J. Characterization of normal and abnormal variants of galactose-1-phosphate uridylyltransferase (EC 2.7.7.12) by isoelectric focusing. Hum Genet. 1983;63(3):274–279. doi: 10.1007/BF00284663. [DOI] [PubMed] [Google Scholar]
- Kelley R. I., Segal S. Evaluation of reduced activity galactose-1-phosphate uridyl transferase by combined radioisotopic assay and high-resolution isoelectric focusing. J Lab Clin Med. 1989 Aug;114(2):152–156. [PubMed] [Google Scholar]
- Komrower G. M., Lee D. H. Long-term follow-up of galactosaemia. Arch Dis Child. 1970 Jun;45(241):367–373. doi: 10.1136/adc.45.241.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnl P., Nowicki L., Spielmann W. Untersuchungen zum Polymorphismus der Galaktose-1-Phosphat-Uridyltransferase (EC: 2.7.7.12) mittels Agarosegelelektrophorese. Humangenetik. 1974;24(3):227–230. [PubMed] [Google Scholar]
- LELOIR L. F. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem Biophys. 1951 Sep;33(2):186–190. doi: 10.1016/0003-9861(51)90096-3. [DOI] [PubMed] [Google Scholar]
- Lee J. E., Ng W. G. Semi-micro techniques for the genotyping of galactokinase and galactose-1-phosphate uridyltransferase. Clin Chim Acta. 1982 Sep 30;124(3):351–356. doi: 10.1016/0009-8981(82)90429-6. [DOI] [PubMed] [Google Scholar]
- Leslie N. D., Immerman E. B., Flach J. E., Florez M., Fridovich-Keil J. L., Elsas L. J. The human galactose-1-phosphate uridyltransferase gene. Genomics. 1992 Oct;14(2):474–480. doi: 10.1016/s0888-7543(05)80244-7. [DOI] [PubMed] [Google Scholar]
- Mellman W. J., Tedesco T. A. An improved assay of erythrocyte and leukocyte galactose-1-phosphate uridyl transferase: stabilization of the enzyme by a thiol protective reagent. J Lab Clin Med. 1965 Dec;66(6):980–986. [PubMed] [Google Scholar]
- Ng W. G., Bergren W. R., Donnell G. N. An improved procedure for the assay of hemolysate galactose-1-phosphate uridyl transferase activity by the use of 14C-labeled galactose-1-phosphate. Clin Chim Acta. 1967 Mar;15(3):489–492. doi: 10.1016/0009-8981(67)90014-9. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Berg P. Cloning and characterization of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1988 Apr;5(2):107–122. [PubMed] [Google Scholar]
- Reichardt J. K., Packman S., Woo S. L. Molecular characterization of two galactosemia mutations: correlation of mutations with highly conserved domains in galactose-1-phosphate uridyl transferase. Am J Hum Genet. 1991 Oct;49(4):860–867. [PMC free article] [PubMed] [Google Scholar]
- Reichardt J. K., Woo S. L. Molecular basis of galactosemia: mutations and polymorphisms in the gene encoding human galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2633–2637. doi: 10.1073/pnas.88.7.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. S., Niedermeier H. P., Endres W., Schaub J., Weidinger S. Agarose gel isoelectrofocusing of UDP-galactose pyrophosphorylase and galactose-1-phosphate uridyltransferase. Developmental aspect of UDP-galactose pyrophosphorylase. Clin Chim Acta. 1987 Jun 30;166(1):27–35. doi: 10.1016/0009-8981(87)90191-4. [DOI] [PubMed] [Google Scholar]
- Sparkes M. C., Crist M., Sparkes R. S. Improved technique for electrophoresis of human galactose-1-p uridyl transferase (EC 2.7.7.12). Hum Genet. 1977 Dec 29;40(1):93–97. doi: 10.1007/BF00280835. [DOI] [PubMed] [Google Scholar]
- Tedesco T. A. Human galactose 1-phosphate uridyltransferase. Purification, antibody production, and comparison of the wild type, Duarte variant, and galactosemic gene products. J Biol Chem. 1972 Oct 25;247(20):6631–6636. [PubMed] [Google Scholar]
- Waggoner D. D., Buist N. R., Donnell G. N. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis. 1990;13(6):802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- Waisbren S. E., Norman T. R., Schnell R. R., Levy H. L. Speech and language deficits in early-treated children with galactosemia. J Pediatr. 1983 Jan;102(1):75–77. doi: 10.1016/s0022-3476(83)80292-3. [DOI] [PubMed] [Google Scholar]