Abstract
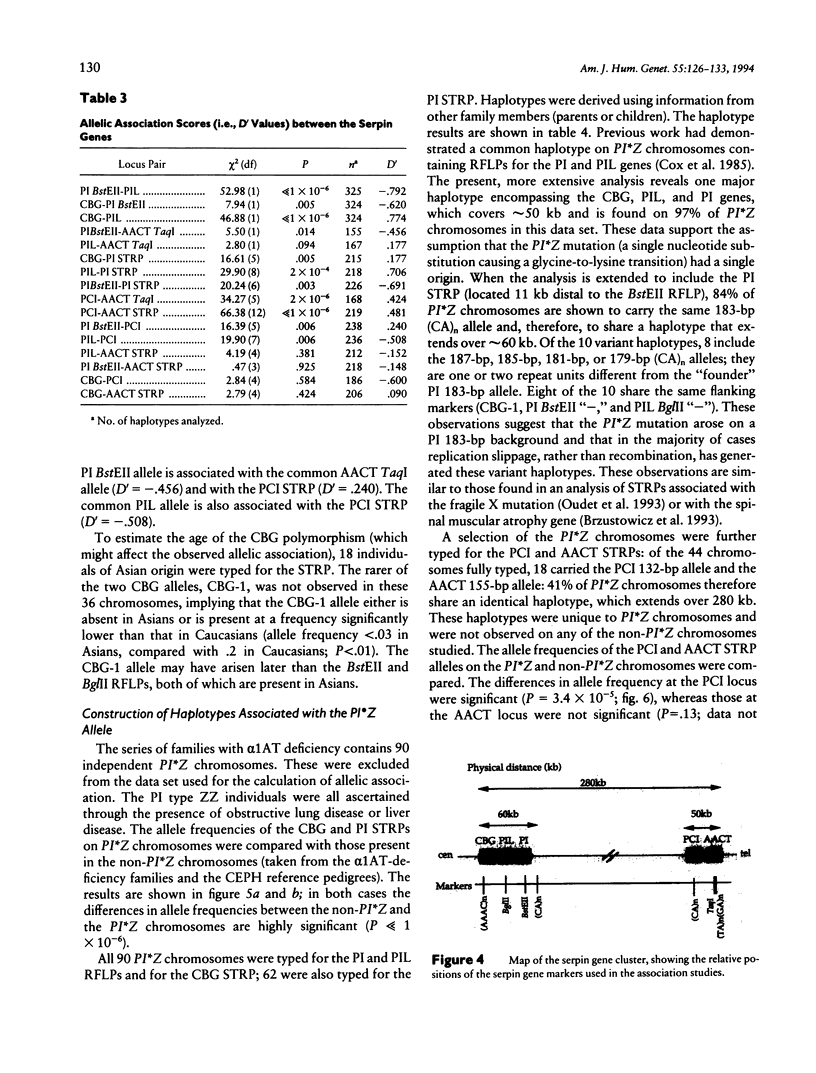
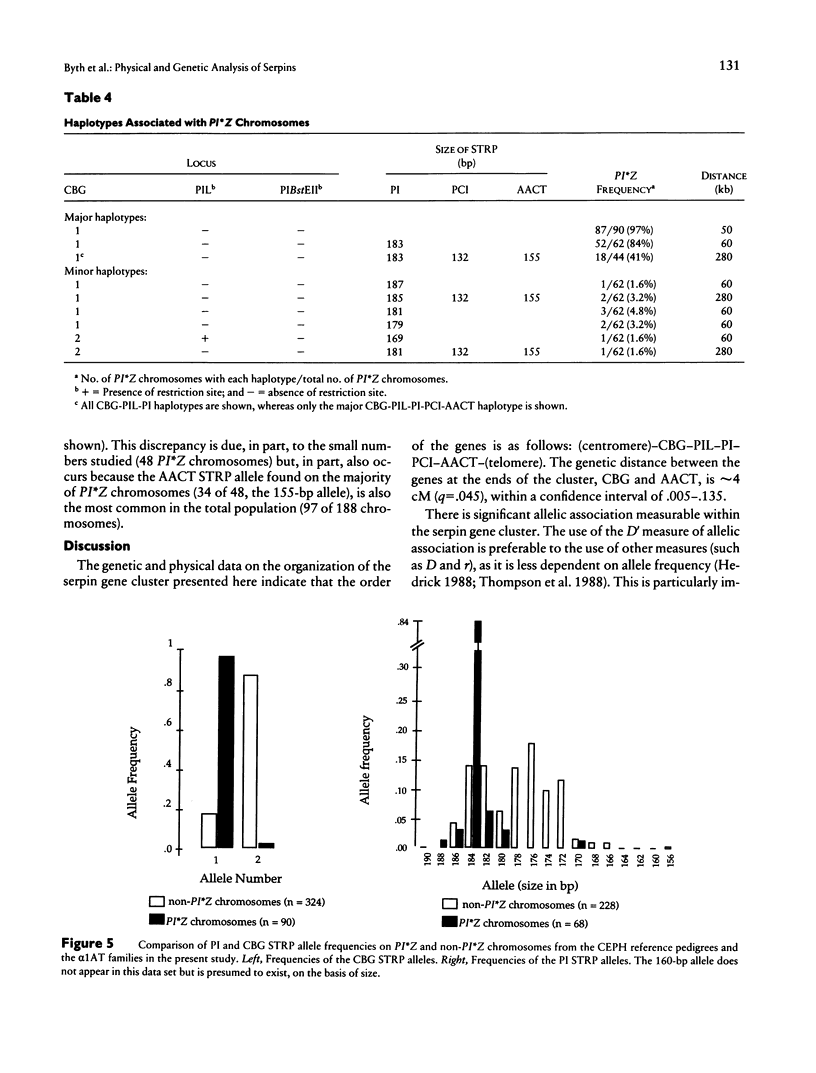
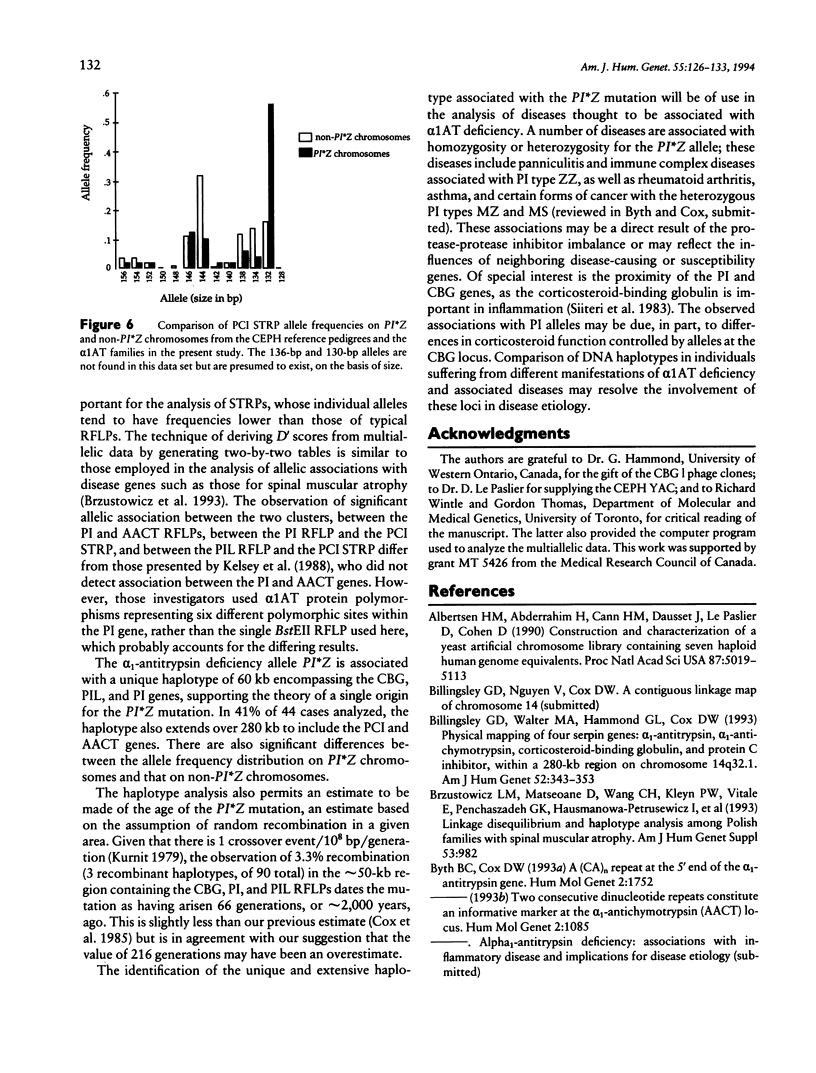
The alpha 1-antitrypsin (PI) gene is part of a cluster of structurally related serine protease inhibitor genes localized at chromosome 14q32.1, a cluster that includes the alpha 1-antichymotrypsin (AACT), protein C inhibitor (PCI), and corticosteroid-binding globulin (CBG) genes and the alpha 1-antitrypsin-like pseudogene (PIL). The order of the genes is refined here by genetic mapping using simple tandem repeat polymorphisms (STRPs) and by physical mapping in YACs. The order of the genes is (centromere)-CBG-PIL-PI-PCI-AACT-(telomere). Analysis of DNA haplotypes comprising STRP and RFLP markers in the serpin genes reveals considerable allelic association throughout the cluster. Furthermore, the common alpha 1-antitrypsin deficiency allele, PI*Z, has a unique DNA haplotype at the CBG, PIL, and PI loci, which extends over 60 kb in 97% of cases and in 44% of cases includes the PCI and AACT loci. This unique haplotype will be of use in examining a number of other diseases, particularly those with an inflammatory component, thought to be associated with alpha 1-antitrypsin deficiency or partial deficiency.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billingsley G. D., Walter M. A., Hammond G. L., Cox D. W. Physical mapping of four serpin genes: alpha 1-antitrypsin, alpha 1-antichymotrypsin, corticosteroid-binding globulin, and protein C inhibitor, within a 280-kb region on chromosome I4q32.1. Am J Hum Genet. 1993 Feb;52(2):343–353. [PMC free article] [PubMed] [Google Scholar]
- Byth B. C., Cox D. W. A (CA)n repeat polymorphism at the 5' end of the alpha 1-antitrypsin gene (PI). Hum Mol Genet. 1993 Oct;2(10):1752–1752. [PubMed] [Google Scholar]
- Byth B. C., Meijers J. C., Cox D. W. A (CA)n repeat polymorphism in the protein C inhibitor (PCI) gene. Hum Mol Genet. 1993 Oct;2(10):1752–1752. [PubMed] [Google Scholar]
- Conneally P. M., Edwards J. H., Kidd K. K., Lalouel J. M., Morton N. E., Ott J., White R. Report of the Committee on Methods of Linkage Analysis and Reporting. Cytogenet Cell Genet. 1985;40(1-4):356–359. doi: 10.1159/000132186. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Woo S. L., Mansfield T. DNA restriction fragments associated with alpha 1-antitrypsin indicate a single origin for deficiency allele PI Z. Nature. 1985 Jul 4;316(6023):79–81. doi: 10.1038/316079a0. [DOI] [PubMed] [Google Scholar]
- Hedrick P. W. Inference of recombinational hotspots using gametic disequilibrium values. Heredity (Edinb) 1988 Jun;60(Pt 3):435–438. doi: 10.1038/hdy.1988.61. [DOI] [PubMed] [Google Scholar]
- Kelsey G. D., Abeliovich D., McMahon C. J., Whitehouse D., Corney G., Povey S., Hopkinson D. A., Wolfe J., Mieli-Vergani G., Mowat A. P. Cloning of the human alpha 1 antichymotrypsin gene and genetic analysis of the gene in relation to alpha 1 antitrypsin deficiency. J Med Genet. 1988 Jun;25(6):361–368. doi: 10.1136/jmg.25.6.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnit D. M. Evolution of sickle variant gene. Lancet. 1979 Jan;1(8107):104–104. doi: 10.1016/s0140-6736(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Lerner T. J., Boustany R. M., MacCormack K., Gleitsman J., Schlumpf K., Breakefield X. O., Gusella J. F., Haines J. L. Linkage disequilibrium between the juvenile neuronal ceroid lipofuscinosis gene and marker loci on chromosome 16p 12.1. Am J Hum Genet. 1994 Jan;54(1):88–94. [PMC free article] [PubMed] [Google Scholar]
- Lewontin R C. The Interaction of Selection and Linkage. I. General Considerations; Heterotic Models. Genetics. 1964 Jan;49(1):49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet C., Mornet E., Serre J. L., Thomas F., Lentes-Zengerling S., Kretz C., Deluchat C., Tejada I., Boué J., Boué A. Linkage disequilibrium between the fragile X mutation and two closely linked CA repeats suggests that fragile X chromosomes are derived from a small number of founder chromosomes. Am J Hum Genet. 1993 Feb;52(2):297–304. [PMC free article] [PubMed] [Google Scholar]
- Sefton L., Kelsey G., Kearney P., Povey S., Wolfe J. A physical map of the human PI and AACT genes. Genomics. 1990 Jul;7(3):382–388. doi: 10.1016/0888-7543(90)90172-q. [DOI] [PubMed] [Google Scholar]
- Sherrington R., Melmer G., Dixon M., Curtis D., Mankoo B., Kalsi G., Gurling H. Linkage disequilibrium between two highly polymorphic microsatellites. Am J Hum Genet. 1991 Nov;49(5):966–971. [PMC free article] [PubMed] [Google Scholar]
- Siiteri P. K., Murai J. T., Hammond G. L., Nisker J. A., Raymoure W. J., Kuhn R. W. The serum transport of steroid hormones. Recent Prog Horm Res. 1982;38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- Thompson E. A., Deeb S., Walker D., Motulsky A. G. The detection of linkage disequilibrium between closely linked markers: RFLPs at the AI-CIII apolipoprotein genes. Am J Hum Genet. 1988 Jan;42(1):113–124. [PMC free article] [PubMed] [Google Scholar]