Abstract
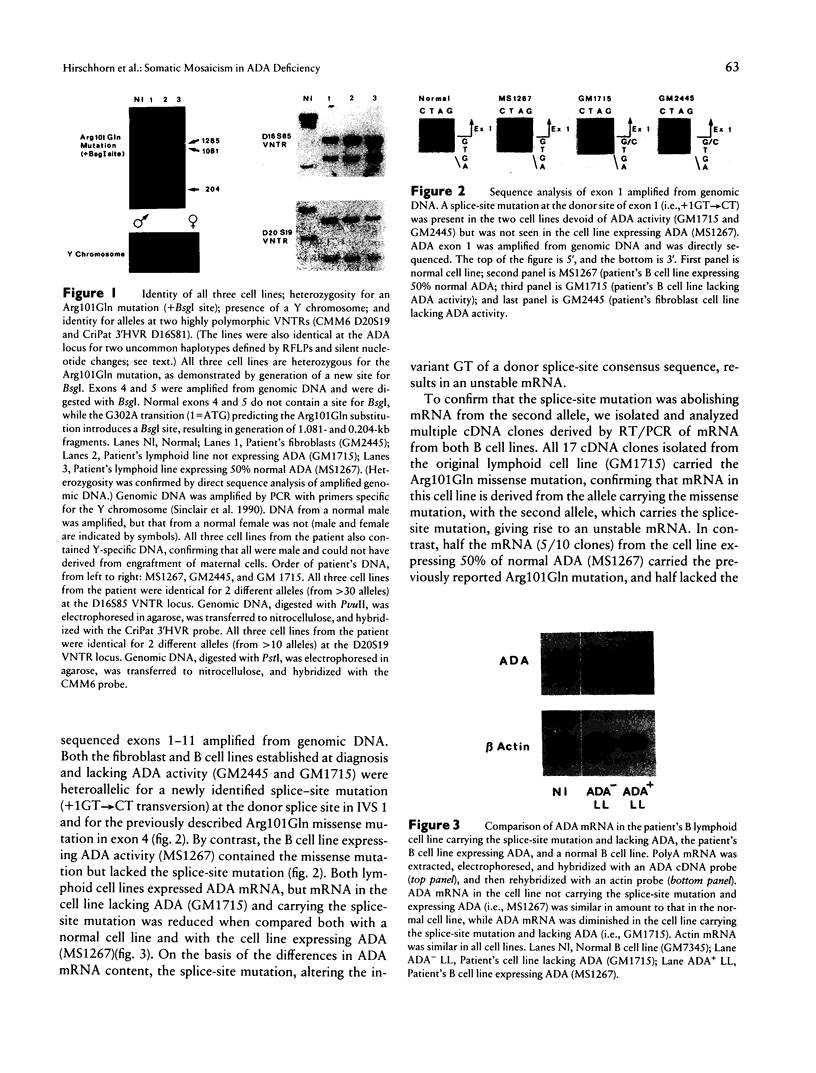
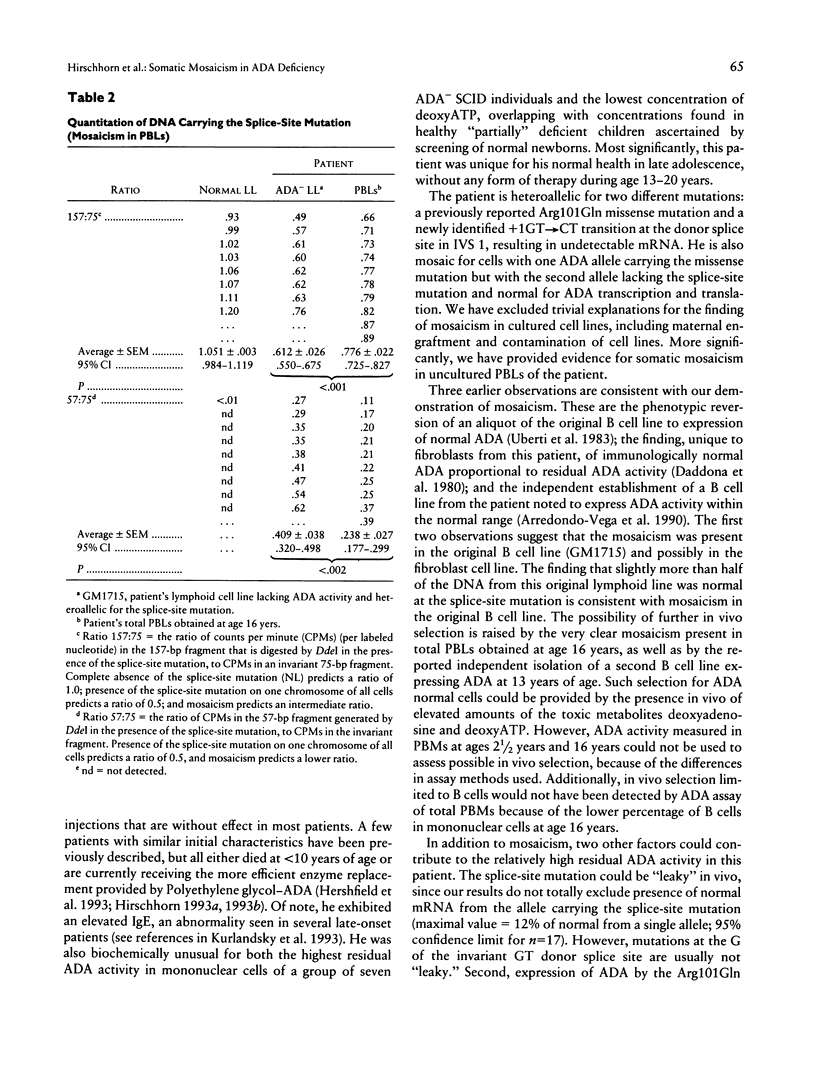
Absent or severely reduced adenosine deaminase (ADA) activity produces inherited immunodeficiency of varying severity, with defects of both cellular and humoral immunity. We report somatic mosaicism as the basis for a delayed presentation and unusual course of a currently healthy young adult receiving no therapy. He was diagnosed at age 2 1/2 years because of life-threatening pneumonia, recurrent infections, failure of normal growth, and lymphopenia, but he retained significant cellular immune function. A fibroblast cell line and a B cell line, established at diagnosis, lacked ADA activity and were heteroallelic for splice-donor-site mutation in IVS 1 (+1GT-->CT) and a missense mutation (Arg101Gln). All clones (17/17) isolated from the B cell mRNA carried the missense mutation, indicating that the allele with the splice-site mutation produced unstable mRNA. In striking contrast, a B cell line established at age 16 years expressed 50% of normal ADA; 50% of ADA mRNA had normal sequence, and 50% had the missense mutation. Genomic DNA contained the missense mutation but not the splice-site mutation. All three cell lines were identical for multiple polymorphic markers and the presence of a Y chromosome. In vivo somatic mosaicism was demonstrated in genomic DNA from peripheral blood cells obtained at 16 years of age, in that less than half the DNA carried the splice-site mutation (P < .002, vs. original B cell line). Consistent with mosaicism, erythrocyte content of the toxic metabolite deoxyATP was only minimally elevated. Somatic mosaicism could have arisen either by somatic mutation or by reversion at the site of mutation. Selection in vivo for ADA normal hematopoietic cells may have played a role in the return to normal health, in the absence of therapy.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeson A. L., Wiginton D. A., Dusing M. R., States J. C., Hutton J. J. Mutant human adenosine deaminase alleles and their expression by transfection into fibroblasts. J Biol Chem. 1988 Nov 5;263(31):16291–16296. [PubMed] [Google Scholar]
- Akeson A. L., Wiginton D. A., States J. C., Perme C. M., Dusing M. R., Hutton J. J. Mutations in the human adenosine deaminase gene that affect protein structure and RNA splicing. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5947–5951. doi: 10.1073/pnas.84.16.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo-Vega F. X., Kurtzberg J., Chaffee S., Santisteban I., Reisner E., Povey M. S., Hershfield M. S. Paradoxical expression of adenosine deaminase in T cells cultured from a patient with adenosine deaminase deficiency and combine immunodeficiency. J Clin Invest. 1990 Aug;86(2):444–452. doi: 10.1172/JCI114730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R., Martiniuk F., Hirschhorn R., Goldschneider I. The distribution of adenosine deaminase among lymphocyte populations in the rat. J Immunol. 1979 Jan;122(1):216–220. [PubMed] [Google Scholar]
- Berkvens T. M., van Ormondt H., Gerritsen E. J., Khan P. M., van der Eb A. J. Identical 3250-bp deletion between two AluI repeats in the ADA genes of unrelated ADA-SCID patients. Genomics. 1990 Aug;7(4):486–490. doi: 10.1016/0888-7543(90)90190-6. [DOI] [PubMed] [Google Scholar]
- Bonthron D. T., Markham A. F., Ginsburg D., Orkin S. H. Identification of a point mutation in the adenosine deaminase gene responsible for immunodeficiency. J Clin Invest. 1985 Aug;76(2):894–897. doi: 10.1172/JCI112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Daddona P. E., Frohman M. A., Kelley W. N. Human adenosine deaminase and its binding protein in normal and adenosine deaminase-deficient fibroblast cell strains. J Biol Chem. 1980 Jun 25;255(12):5681–5687. [PubMed] [Google Scholar]
- Daddona P. E., Kelley W. N. Characteristics of an aminohydrolase distinct from adenosine deaminase in cultured human lymphoblasts. Biochim Biophys Acta. 1981 Apr 14;658(2):280–290. doi: 10.1016/0005-2744(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Shewach D. S., Kelley W. N., Argos P., Markham A. F., Orkin S. H. Human adenosine deaminase. cDNA and complete primary amino acid sequence. J Biol Chem. 1984 Oct 10;259(19):12101–12106. [PubMed] [Google Scholar]
- Hall J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet. 1988 Oct;43(4):355–363. [PMC free article] [PubMed] [Google Scholar]
- Hershfield M. S., Chaffee S., Sorensen R. U. Enzyme replacement therapy with polyethylene glycol-adenosine deaminase in adenosine deaminase deficiency: overview and case reports of three patients, including two now receiving gene therapy. Pediatr Res. 1993 Jan;33(1 Suppl):S42–S48. doi: 10.1203/00006450-199305001-00236. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Chakravarti V., Puck J., Douglas S. D. Homozygosity for a newly identified missense mutation in a patient with very severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID). Am J Hum Genet. 1991 Oct;49(4):878–885. [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Chen A. S., Israni A., Yang D. R., Huie M. L. Two new mutations at the adenosine deaminase (ADA) locus (Q254X and del nt1050-54) unusual for not being missense mutations. Hum Mutat. 1993;2(4):320–323. doi: 10.1002/humu.1380020415. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Ellenbogen A., Tzall S. Five missense mutations at the adenosine deaminase locus (ADA) detected by altered restriction fragments and their frequency in ADA--patients with severe combined immunodeficiency (ADA-SCID). Am J Med Genet. 1992 Jan 15;42(2):201–207. doi: 10.1002/ajmg.1320420213. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. Identification of two new missense mutations (R156C and S291L) in two ADA- SCID patients unusual for response to therapy with partial exchange transfusions. Hum Mutat. 1992;1(2):166–168. doi: 10.1002/humu.1380010214. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Nicknam M. N., Eng F., Yang D. R., Borkowsky W. Novel deletion and a new missense mutation (Glu 217 Lys) at the catalytic site in two adenosine deaminase alleles of a patient with neonatal onset adenosine deaminase- severe combined immunodeficiency. J Immunol. 1992 Nov 1;149(9):3107–3112. [PubMed] [Google Scholar]
- Hirschhorn R., Ratech H., Rubinstein A., Papageorgiou P., Kesarwala H., Gelfand E., Roegner-Maniscalco V. Increased excretion of modified adenine nucleosides by children with adenosine deaminase deficiency. Pediatr Res. 1982 May;16(5):362–369. doi: 10.1203/00006450-198205000-00009. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Roegner-Maniscalco V., Kuritsky L., Rosen F. S. Bone marrow transplantation only partially restores purine metabolites to normal in adenosine deaminase-deficient patients. J Clin Invest. 1981 Dec;68(6):1387–1393. doi: 10.1172/JCI110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Roegner V., Jenkins T., Seaman C., Piomelli S., Borkowsky W. Erythrocyte adenosine deaminase deficiency without immunodeficiency. Evidence for an unstable mutant enzyme. J Clin Invest. 1979 Oct;64(4):1130–1139. doi: 10.1172/JCI109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Tzall S., Ellenbogen A. Hot spot mutations in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6171–6175. doi: 10.1073/pnas.87.16.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Tzall S., Ellenbogen A., Orkin S. H. Identification of a point mutation resulting in a heat-labile adenosine deaminase (ADA) in two unrelated children with partial ADA deficiency. J Clin Invest. 1989 Feb;83(2):497–501. doi: 10.1172/JCI113909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Yang D. R., Insel R. A., Ballow M. Severe combined immunodeficiency of reduced severity due to homozygosity for an adenosine deaminase missense mutation (Arg253Pro). Cell Immunol. 1993 Dec;152(2):383–393. doi: 10.1006/cimm.1993.1299. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Yang D. R., Israni A. An Asp8Asn substitution results in the adenosine deaminase (ADA) genetic polymorphism (ADA 2 allozyme): occurrence on different chromosomal backgrounds and apparent intragenic crossover. Ann Hum Genet. 1994 Jan;58(Pt 1):1–9. [PubMed] [Google Scholar]
- Hopkinson D. A., Cook P. J., Harris H. Further data on the adenosine deaminase (ADA) polymprphism and a report of a new phenotype. Ann Hum Genet. 1969 May;32(4):361–367. doi: 10.1111/j.1469-1809.1969.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto H., Ito K., Kashii S., Monden S., Fujita M., Norioka M., Sasai Y., Okuma M. A point mutation in the 5' splice region of intron 7 causes a deletion of exon 7 in adenosine deaminase mRNA. J Cell Biochem. 1993 Mar;51(3):322–325. doi: 10.1002/jcb.240510311. [DOI] [PubMed] [Google Scholar]
- Keith T. P., Green P., Reeders S. T., Brown V. A., Phipps P., Bricker A., Falls K., Rediker K. S., Powers J. A., Hogan C. Genetic linkage map of 46 DNA markers on human chromosome 16. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5754–5758. doi: 10.1073/pnas.87.15.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd K. K., Bowcock A. M., Schmidtke J., Track R. K., Ricciuti F., Hutchings G., Bale A., Pearson P., Willard H. F., Gelernter J. Report of the DNA committee and catalogs of cloned and mapped genes and DNA polymorphisms. Cytogenet Cell Genet. 1989;51(1-4):622–947. doi: 10.1159/000132810. [DOI] [PubMed] [Google Scholar]
- Levinson B., Lehesjoki A. E., de la Chapelle A., Gitschier J. Molecular analysis of hemophilia A mutations in the Finnish population. Am J Hum Genet. 1990 Jan;46(1):53–62. [PMC free article] [PubMed] [Google Scholar]
- Maddalena A., Sosnoski D. M., Berry G. T., Nussbaum R. L. Mosaicism for an intragenic deletion in a boy with mild ornithine transcarbamylase deficiency. N Engl J Med. 1988 Oct 13;319(15):999–1003. doi: 10.1056/NEJM198810133191507. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Norby-Slycord C., Ward F. E. A high proportion of ADA point mutations associated with a specific alanine-to-valine substitution. Am J Hum Genet. 1989 Sep;45(3):354–361. [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Martin C., Leppert M., O'Connell P., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pCMM6) on chromosome 20 [D20S19]. Nucleic Acids Res. 1988 Jun 10;16(11):5222–5222. doi: 10.1093/nar/16.11.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polmar S. H., Stern R. C., Schwartz A. L., Wetzler E. M., Chase P. A., Hirschhorn R. Enzyme replacement therapy for adenosine deaminase deficiency and severe combined immunodeficiency. N Engl J Med. 1976 Dec 9;295(24):1337–1343. doi: 10.1056/NEJM197612092952402. [DOI] [PubMed] [Google Scholar]
- Ratech H., Thorbecke G. J., Meredith G., Hirschhorn R. Comparison and possible homology of isozymes of adenosine deaminase in Aves and humans. Enzyme. 1981;26(2):74–84. doi: 10.1159/000459153. [DOI] [PubMed] [Google Scholar]
- Schwindinger W. F., Francomano C. A., Levine M. A. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5152–5156. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shovlin C. L., Hughes J. M., Simmonds H. A., Fairbanks L., Deacock S., Lechler R., Roberts I., Webster A. D. Adult presentation of adenosine deaminase deficiency. Lancet. 1993 Jun 5;341(8858):1471–1471. doi: 10.1016/0140-6736(93)90910-9. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. A., Deugau K. V., Lillicrap D. P. Somatic mosaicism and female-to-female transmission in a kindred with hemophilia B (factor IX deficiency). Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):39–42. doi: 10.1073/pnas.88.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung R., Silber R., Quagliata F., Conklyn M., Gottesman J., Hirschhorn R. Adenosine deaminase activity in chronic lymphocytic leukemia. Relationship to B- and T-cell subpopulations. J Clin Invest. 1976 Mar;57(3):756–761. doi: 10.1172/JCI108334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzall S., Ellenbogen A., Eng F., Hirschhorn R. Identification and characterization of nine RFLPs at the adenosine deaminase (ADA) locus. Am J Hum Genet. 1989 Jun;44(6):864–875. [PMC free article] [PubMed] [Google Scholar]
- Uberti J., Lightbody J. J., Wolf J. W., Anderson J. A., Reid R. H., Johnson R. M. The effect of adenosine on mitogenesis of ADA-deficient lymphocytes. Clin Immunol Immunopathol. 1978 Aug;10(4):446–458. doi: 10.1016/0090-1229(78)90157-5. [DOI] [PubMed] [Google Scholar]
- Uberti J., Peterson W. D., Jr, Lightbody J. J., Johnson R. M. A phenotypically normal revertant of an adenosine deaminase-deficient lymphoblast cell line. J Immunol. 1983 Jun;130(6):2866–2870. [PubMed] [Google Scholar]
- Valerio D., Dekker B. M., Duyvesteyn M. G., van der Voorn L., Berkvens T. M., van Ormondt H., van der Eb A. J. One adenosine deaminase allele in a patient with severe combined immunodeficiency contains a point mutation abolishing enzyme activity. EMBO J. 1986 Jan;5(1):113–119. doi: 10.1002/j.1460-2075.1986.tb04184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio D., McIvor R. S., Williams S. R., Duyvesteyn M. G., van Ormondt H., van der Eb A. J., Martin D. W., Jr Cloning of human adenosine deaminase cDNA and expression in mouse cells. Gene. 1984 Nov;31(1-3):147–153. doi: 10.1016/0378-1119(84)90205-1. [DOI] [PubMed] [Google Scholar]
- Voit T., Neuen-Jacob E., Mahler V., Jauch A., Cremer M. Somatic mosaicism for a deletion of the dystrophin gene in a carrier of Becker muscular dystrophy. Eur J Pediatr. 1992 Feb;151(2):112–116. doi: 10.1007/BF01958954. [DOI] [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Zinn A. B., Byers P. H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990 Jun;46(6):1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Hutton J. J. Sequence of human adenosine deaminase cDNA including the coding region and a small intron. Nucleic Acids Res. 1984 Mar 12;12(5):2439–2446. doi: 10.1093/nar/12.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]
- Yang D. R., Huie M. L., Hirschhorn R. Homozygosity for a missense mutation (G20R) associated with neonatal onset adenosine deaminase-deficient severe combined immunodeficiency (ADA-SCID). Clin Immunol Immunopathol. 1994 Feb;70(2):171–175. doi: 10.1006/clin.1994.1026. [DOI] [PubMed] [Google Scholar]