Abstract
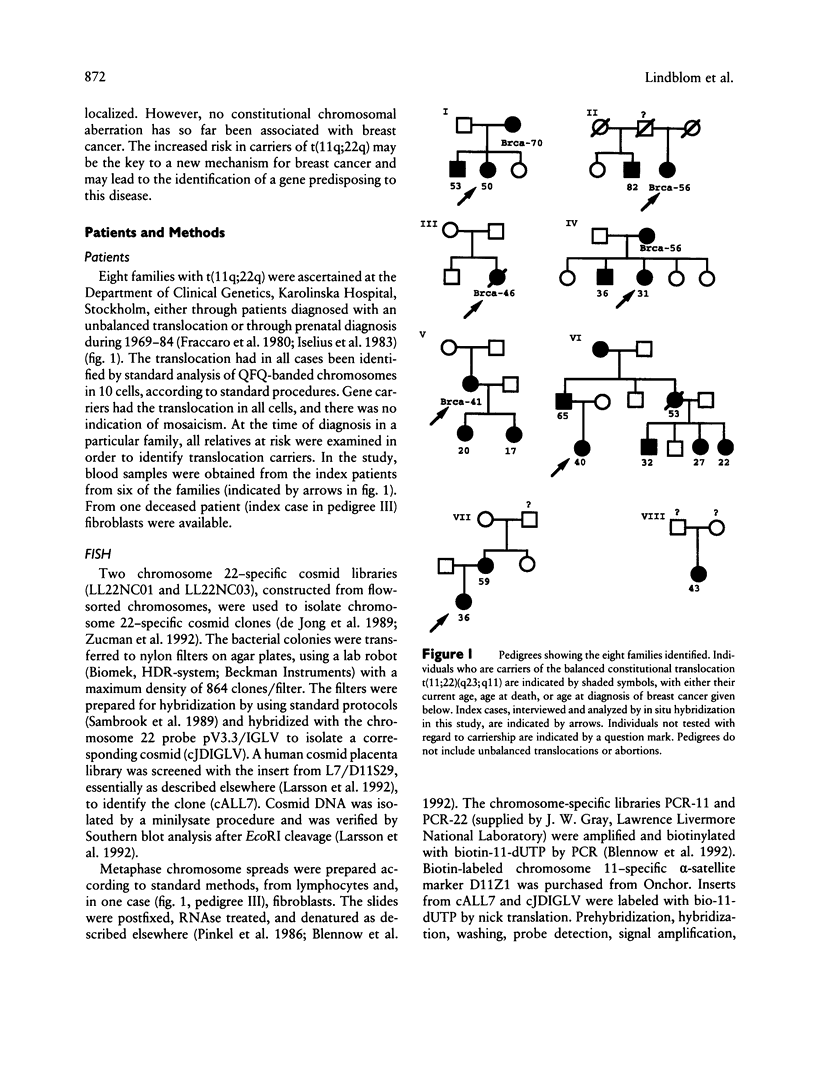
A translocation between the long arms of chromosomes 11 and 22, t(11;22)(q23;q11), is the most frequent constitutional reciprocal translocation in man. This chromosome abnormality has not previously been reported to be associated with an increased risk for neoplasia. The observation of one patient with a constitutional translocation t(11q;22q) and breast cancer prompted us to study the relationship between these two conditions. The incidence of breast cancer was determined in carriers of t(11q;22q). The karyotypes were determined by QFQ-banding, and the breakpoints were then further characterized by fluorescent in situ hybridization. Eight families with a total of 22 balanced carriers were found. In five of these families there was one case of breast cancer each. In another family a case of an unknown malignancy was reported in one member. No other malignancies were found among these patients. The number of breast cancer cases was significantly higher than expected among the translocation carriers (P < .001). The chromosomal breakpoints showed the same localization with the markers used, in the seven families studied. The association of constitutional translocation t(11q;22q) and breast cancer identifies a subset of patients with a highly increased risk for breast cancer who would benefit from counseling and screening. It also suggests the involvement of genes on 11q and/or 22q, in the tumorigenesis of breast cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blennow E., Telenius H., Larsson C., de Vos D., Bajalica S., Ponder B. A., Nordenskjöld M. Complete characterization of a large marker chromosome by reverse and forward chromosome painting. Hum Genet. 1992 Dec;90(4):371–374. doi: 10.1007/BF00220461. [DOI] [PubMed] [Google Scholar]
- Cawthon R. M., Weiss R., Xu G. F., Viskochil D., Culver M., Stevens J., Robertson M., Dunn D., Gesteland R., O'Connell P. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990 Jul 13;62(1):193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- Cohen A. J., Li F. P., Berg S., Marchetto D. J., Tsai S., Jacobs S. C., Brown R. S. Hereditary renal-cell carcinoma associated with a chromosomal translocation. N Engl J Med. 1979 Sep 13;301(11):592–595. doi: 10.1056/NEJM197909133011107. [DOI] [PubMed] [Google Scholar]
- Cremer T., Lichter P., Borden J., Ward D. C., Manuelidis L. Detection of chromosome aberrations in metaphase and interphase tumor cells by in situ hybridization using chromosome-specific library probes. Hum Genet. 1988 Nov;80(3):235–246. doi: 10.1007/BF01790091. [DOI] [PubMed] [Google Scholar]
- Curran M. E., Atkinson D. L., Ewart A. K., Morris C. A., Leppert M. F., Keating M. T. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993 Apr 9;73(1):159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- Ferti-Passantonopoulou A., Panani A. D., Raptis S. Preferential involvement of 11q23-24 and 11p15 in breast cancer. Cancer Genet Cytogenet. 1991 Feb;51(2):183–188. doi: 10.1016/0165-4608(91)90130-m. [DOI] [PubMed] [Google Scholar]
- Fraccaro M., Lindsten J., Ford C. E., Iselius L. The 11q;22q translocation: a European collaborative analysis of 43 cases. Hum Genet. 1980;56(1):21–51. doi: 10.1007/BF00281567. [DOI] [PubMed] [Google Scholar]
- Griffin C. A., McKeon C., Israel M. A., Gegonne A., Ghysdael J., Stehelin D., Douglass E. C., Green A. E., Emanuel B. S. Comparison of constitutional and tumor-associated 11;22 translocations: nonidentical breakpoints on chromosomes 11 and 22. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6122–6126. doi: 10.1073/pnas.83.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Iselius L., Lindsten J., Aurias A., Fraccaro M., Bastard C., Bottelli A. M., Bui T. H., Caufin D., Dalprà L., Delendi N. The 11q;22q translocation: a collaborative study of 20 new cases and analysis of 110 families. Hum Genet. 1983;64(4):343–355. doi: 10.1007/BF00292366. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Lambert C., Schultz R. A., Smith M., Wagner-McPherson C., McDaniel L. D., Donlon T., Stanbridge E. J., Friedberg E. C. Functional complementation of ataxia-telangiectasia group D (AT-D) cells by microcell-mediated chromosome transfer and mapping of the AT-D locus to the region 11q22-23. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5907–5911. doi: 10.1073/pnas.88.13.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Weber G., Kvanta E., Lewis K., Janson M., Jones C., Glaser T., Evans G., Nordenskjöld M. Isolation and mapping of polymorphic cosmid clones used for sublocalization of the multiple endocrine neoplasia type 1 (MEN1) locus. Hum Genet. 1992 May;89(2):187–193. doi: 10.1007/BF00217121. [DOI] [PubMed] [Google Scholar]
- Ledbetter D. H., Rich D. C., O'Connell P., Leppert M., Carey J. C. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989 Jan;44(1):20–24. [PMC free article] [PubMed] [Google Scholar]
- Lindblom A., Skoog L., Rotstein S., Werelius B., Larsson C., Nordenskjöld M. Loss of heterozygosity in familial breast carcinomas. Cancer Res. 1993 Sep 15;53(18):4356–4361. [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Menon A. G., Ledbetter D. H., Rich D. C., Seizinger B. R., Rouleau G. A., Michels V. F., Schmidt M. A., Dewald G., DallaTorre C. M., Haines J. L. Characterization of a translocation within the von Recklinghausen neurofibromatosis region of chromosome 17. Genomics. 1989 Aug;5(2):245–249. doi: 10.1016/0888-7543(89)90053-0. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Morrell D., Chase C. L., Swift M. Cancers in 44 families with ataxia-telangiectasia. Cancer Genet Cytogenet. 1990 Nov 1;50(1):119–123. doi: 10.1016/0165-4608(90)90245-6. [DOI] [PubMed] [Google Scholar]
- Motegi T., Komatsu M., Nakazato Y., Ohuchi M., Minoda K. Retinoblastoma in a boy with a de novo mutation of a 13/18 translocation: the assumption that the retinoblastoma locus is at 13q141, particularly at the distal portion of it. Hum Genet. 1982;60(2):193–195. doi: 10.1007/BF00569711. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Feunteun J., Lynch H. T., Watson P., Conway T., Lynch J., Lenoir G. M. Familial breast-ovarian cancer locus on chromosome 17q12-q23. Lancet. 1991 Jul 13;338(8759):82–83. doi: 10.1016/0140-6736(91)90076-2. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Zou Z. Q., Pirollo K., Blattner W., Chang E. H. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990 Dec 20;348(6303):747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- Turleau C., de Grouchy J., Chavin-Colin F., Junien C., Séger J., Schlienger P., Leblanc A., Haye C. Cytogenetic forms of retinoblastoma: their incidence in a survey of 66 patients. Cancer Genet Cytogenet. 1985 Apr 15;16(4):321–334. doi: 10.1016/0165-4608(85)90240-7. [DOI] [PubMed] [Google Scholar]
- Viskochil D., Buchberg A. M., Xu G., Cawthon R. M., Stevens J., Wolff R. K., Culver M., Carey J. C., Copeland N. G., Jenkins N. A. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990 Jul 13;62(1):187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Marchuk D. A., Andersen L. B., Letcher R., Odeh H. M., Saulino A. M., Fountain J. W., Brereton A., Nicholson J., Mitchell A. L. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990 Jul 13;249(4965):181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Wooster R., Mangion J., Eeles R., Smith S., Dowsett M., Averill D., Barrett-Lee P., Easton D. F., Ponder B. A., Stratton M. R. A germline mutation in the androgen receptor gene in two brothers with breast cancer and Reifenstein syndrome. Nat Genet. 1992 Oct;2(2):132–134. doi: 10.1038/ng1092-132. [DOI] [PubMed] [Google Scholar]
- Zackai E. H., Emanuel B. S. Site-specific reciprocal translocation, t(11;22) (q23;q11), in several unrelated families with 3:1 meiotic disjunction. Am J Med Genet. 1980;7(4):507–521. doi: 10.1002/ajmg.1320070412. [DOI] [PubMed] [Google Scholar]
- Zucman J., Delattre O., Desmaze C., Plougastel B., Joubert I., Melot T., Peter M., De Jong P., Rouleau G., Aurias A. Cloning and characterization of the Ewing's sarcoma and peripheral neuroepithelioma t(11;22) translocation breakpoints. Genes Chromosomes Cancer. 1992 Nov;5(4):271–277. doi: 10.1002/gcc.2870050402. [DOI] [PubMed] [Google Scholar]



