Abstract
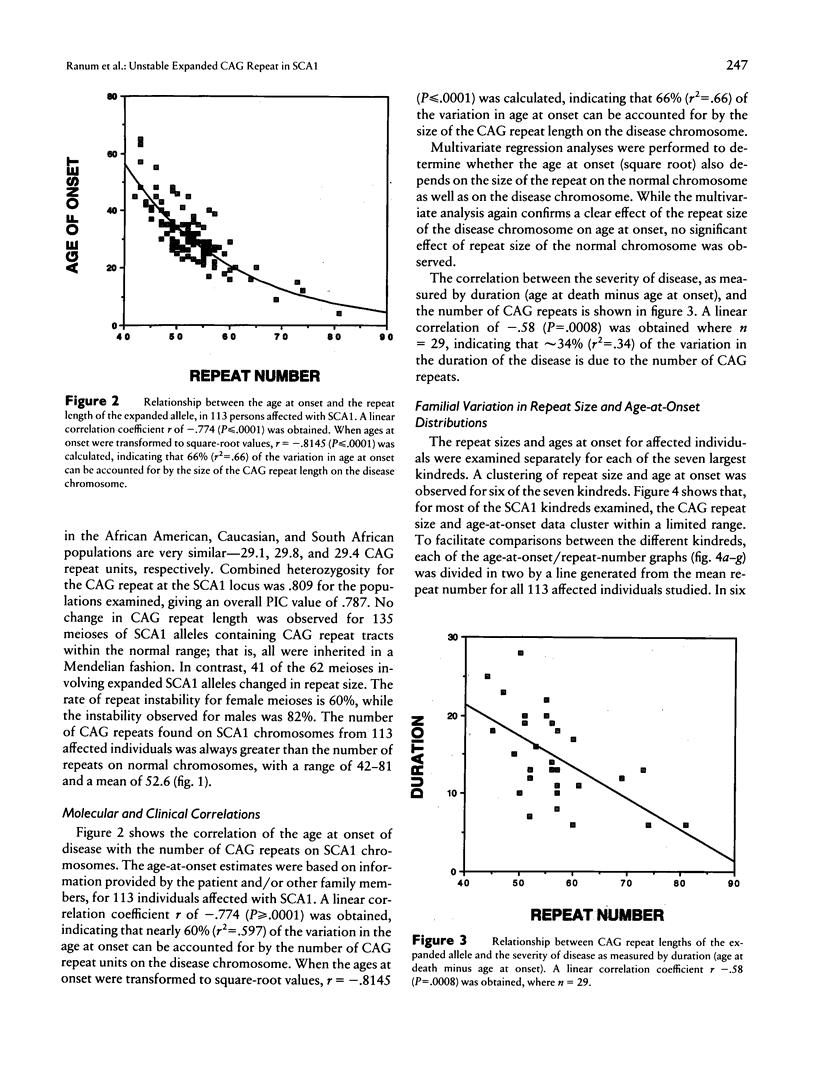
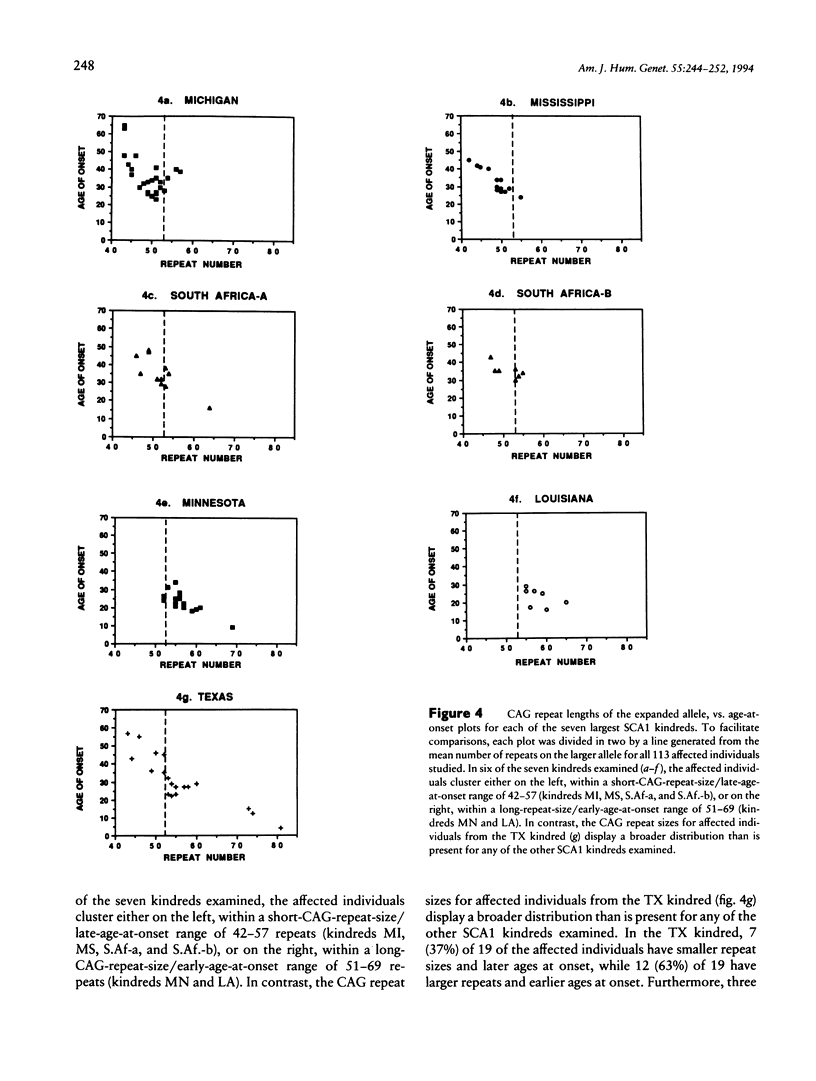
The spinocerebellar ataxias are a group of debilitating neurodegenerative diseases for which a clinical classification system has proved unreliable. We have recently isolated the gene for spinocerebellar ataxia type 1 (SCA1) and have shown that the disease is caused by an expanded, unstable, CAG trinucleotide repeat within an expressed gene. Normal alleles have a size range of 19–36 repeats, while SCA1 alleles have 42-81 repeats. In this study, we examined the frequency and variability of the SCA1 repeat expansion in 87 kindreds with diverse ethnic backgrounds and dominantly inherited ataxia. All nine families for which linkage to the SCA1 region of 6p had previously been established showed repeat expansion, while 3 of the remaining 78 showed a similar abnormality. For 113 patients from the families with repeat expansion, inverse correlations between CAG repeat size and both age at onset and disease duration were observed. Repeat size accounted for 66% of the variation in age at onset in these patients. After correction for repeat size, interfamilial differences in age at onset remained significant, suggesting that additional genetic factors affect the expression of the SCA1 gene product.
Full text
PDF

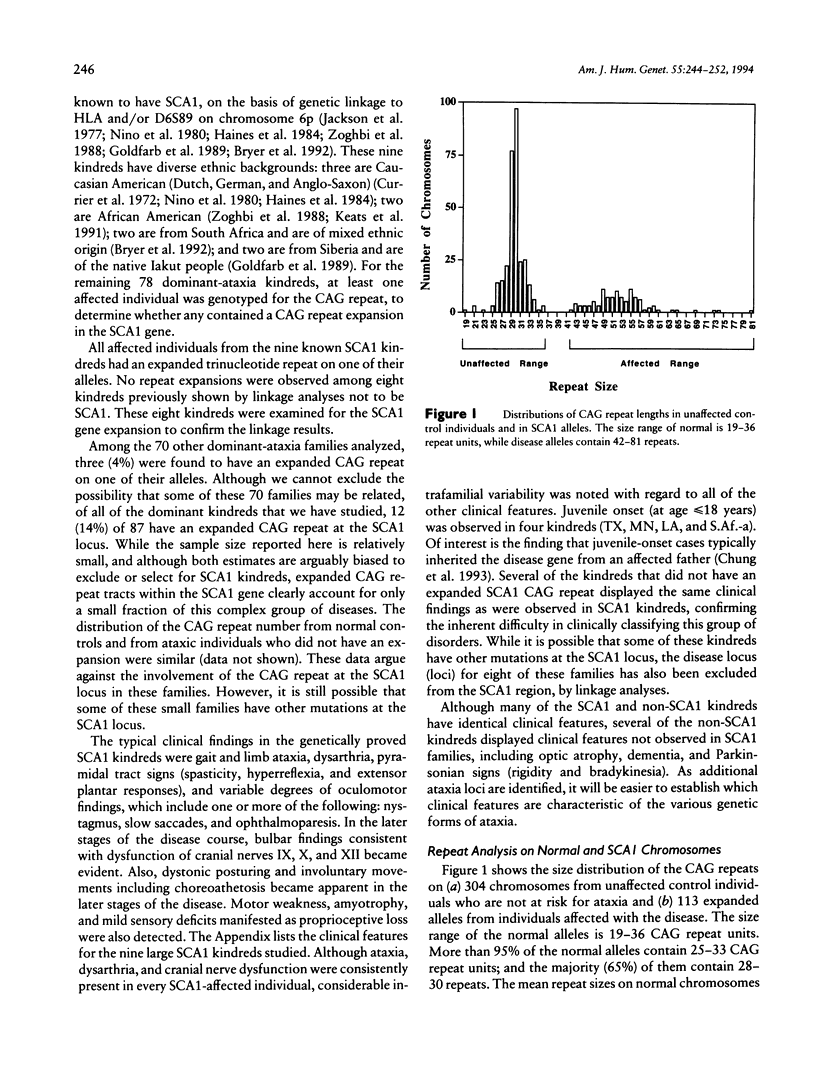




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew S. E., Goldberg Y. P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M. A. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet. 1993 Aug;4(4):398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- Auburger G., Diaz G. O., Capote R. F., Sanchez S. G., Perez M. P., del Cueto M. E., Meneses M. G., Farrall M., Williamson R., Chamberlain S. Autosomal dominant ataxia: genetic evidence for locus heterogeneity from a Cuban founder-effect population. Am J Hum Genet. 1990 Jun;46(6):1163–1177. [PMC free article] [PubMed] [Google Scholar]
- Banfi S., Chung M. Y., Kwiatkowski T. J., Jr, Ranum L. P., McCall A. E., Chinault A. C., Orr H. T., Zoghbi H. Y. Mapping and cloning of the critical region for the spinocerebellar ataxia type 1 gene (SCA1) in a yeast artificial chromosome contig spanning 1.2 Mb. Genomics. 1993 Dec;18(3):627–635. doi: 10.1016/s0888-7543(05)80365-9. [DOI] [PubMed] [Google Scholar]
- Biancalana V., Serville F., Pommier J., Julien J., Hanauer A., Mandel J. L. Moderate instability of the trinucleotide repeat in spino bulbar muscular atrophy. Hum Mol Genet. 1992 Jul;1(4):255–258. doi: 10.1093/hmg/1.4.255. [DOI] [PubMed] [Google Scholar]
- Bryer A., Martell R. W., du Toit E. D., Beighton P. Adult onset spinocerebellar ataxia linked to HLA in a South African kindred of mixed ancestry. Tissue Antigens. 1992 Sep;40(3):111–115. doi: 10.1111/j.1399-0039.1992.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Buxton J., Shelbourne P., Davies J., Jones C., Van Tongeren T., Aslanidis C., de Jong P., Jansen G., Anvret M., Riley B. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature. 1992 Feb 6;355(6360):547–548. doi: 10.1038/355547a0. [DOI] [PubMed] [Google Scholar]
- Carson W. J., Radvany J., Farrer L. A., Vincent D., Rosenberg R. N., MacLeod P. M., Rouleau G. A. The Machado-Joseph disease locus is different from the spinocerebellar ataxia locus (SCA1). Genomics. 1992 Jul;13(3):852–855. doi: 10.1016/0888-7543(92)90168-r. [DOI] [PubMed] [Google Scholar]
- Chung M. Y., Ranum L. P., Duvick L. A., Servadio A., Zoghbi H. Y., Orr H. T. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet. 1993 Nov;5(3):254–258. doi: 10.1038/ng1193-254. [DOI] [PubMed] [Google Scholar]
- Coutinho P., Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978 Jul;28(7):703–709. doi: 10.1212/wnl.28.7.703. [DOI] [PubMed] [Google Scholar]
- Currier R. D., Glover G., Jackson J. F., Tipton A. C. Spinocerebellar ataxia: study of a large kindred. I. General information and genetics. Neurology. 1972 Oct;22(10):1040–1043. doi: 10.1212/wnl.22.9.1040. [DOI] [PubMed] [Google Scholar]
- Diagnosing the heart of the problem. Nat Genet. 1993 Jul;4(3):211–212. doi: 10.1038/ng0793-211. [DOI] [PubMed] [Google Scholar]
- Duyao M., Ambrose C., Myers R., Novelletto A., Persichetti F., Frontali M., Folstein S., Ross C., Franz M., Abbott M. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat Genet. 1993 Aug;4(4):387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gispert S., Twells R., Orozco G., Brice A., Weber J., Heredero L., Scheufler K., Riley B., Allotey R., Nothers C. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet. 1993 Jul;4(3):295–299. doi: 10.1038/ng0793-295. [DOI] [PubMed] [Google Scholar]
- Goldberg Y. P., Kremer B., Andrew S. E., Theilmann J., Graham R. K., Squitieri F., Telenius H., Adam S., Sajoo A., Starr E. Molecular analysis of new mutations for Huntington's disease: intermediate alleles and sex of origin effects. Nat Genet. 1993 Oct;5(2):174–179. doi: 10.1038/ng1093-174. [DOI] [PubMed] [Google Scholar]
- Goldfarb L. G., Chumakov M. P., Petrov P. A., Fedorova N. I., Gajdusek D. C. Olivopontocerebellar atrophy in a large Iakut kinship in eastern Siberia. Neurology. 1989 Nov;39(11):1527–1530. doi: 10.1212/wnl.39.11.1527. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Rundle S. A., Crow S., Reardon W., Buckler A. J., Harper P. S., Housman D. E., Shaw D. J. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992 Feb 6;355(6360):545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- Jackson J. F., Currier R. D., Terasaki P. I., Morton N. E. Spinocerebellar ataxia and HLA linkage: risk prediction by HLA typing. N Engl J Med. 1977 May 19;296(20):1138–1141. doi: 10.1056/NEJM197705192962003. [DOI] [PubMed] [Google Scholar]
- Keats B. J., Pollack M. S., McCall A., Wilensky M. A., Ward L. J., Lu M., Zoghbi H. Y. Tight linkage of the gene for spinocerebellar ataxia to D6S89 on the short arm of chromosome 6 in a kindred for which close linkage to both HLA and F13A1 is excluded. Am J Hum Genet. 1991 Nov;49(5):972–977. [PMC free article] [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- Kremer E. J., Pritchard M., Lynch M., Yu S., Holman K., Baker E., Warren S. T., Schlessinger D., Sutherland G. R., Richards R. I. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991 Jun 21;252(5013):1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- Kumar D., Blank C. E., Gelsthorpe K. Hereditary cerebellar ataxia and genetic linkage with HLA. Hum Genet. 1986 Apr;72(4):327–332. doi: 10.1007/BF00290959. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski T. J., Jr, Orr H. T., Banfi S., McCall A. E., Jodice C., Persichetti F., Novelletto A., LeBorgne-DeMarquoy F., Duvick L. A., Frontali M. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps centromeric to D6S89 and shows no recombination, in nine large kindreds, with a dinucleotide repeat at the AM10 locus. Am J Hum Genet. 1993 Aug;53(2):391–400. [PMC free article] [PubMed] [Google Scholar]
- Li S. H., McInnis M. G., Margolis R. L., Antonarakis S. E., Ross C. A. Novel triplet repeat containing genes in human brain: cloning, expression, and length polymorphisms. Genomics. 1993 Jun;16(3):572–579. doi: 10.1006/geno.1993.1232. [DOI] [PubMed] [Google Scholar]
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992 Mar 6;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Yanagisawa H., Sato K., Shirayama T., Ohsaki E., Bundo M., Takeda T., Tadokoro K., Kondo I., Murayama N. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet. 1994 Jan;6(1):14–18. doi: 10.1038/ng0194-14. [DOI] [PubMed] [Google Scholar]
- Nino H. E., Noreen H. J., Dubey D. P., Resch J. A., Namboodiri K., Elston R. C., Yunis E. J. A family with hereditary ataxia: HLA typing. Neurology. 1980 Jan;30(1):12–20. doi: 10.1212/wnl.30.1.12. [DOI] [PubMed] [Google Scholar]
- Nørremølle A., Riess O., Epplen J. T., Fenger K., Hasholt L., Sørensen S. A. Trinucleotide repeat elongation in the Huntingtin gene in Huntington disease patients from 71 Danish families. Hum Mol Genet. 1993 Sep;2(9):1475–1476. doi: 10.1093/hmg/2.9.1475. [DOI] [PubMed] [Google Scholar]
- Orozco Diaz G., Nodarse Fleites A., Cordovés Sagaz R., Auburger G. Autosomal dominant cerebellar ataxia: clinical analysis of 263 patients from a homogeneous population in Holguín, Cuba. Neurology. 1990 Sep;40(9):1369–1375. doi: 10.1212/wnl.40.9.1369. [DOI] [PubMed] [Google Scholar]
- Ranum L. P., Duvick L. A., Rich S. S., Schut L. J., Litt M., Orr H. T. Localization of the autosomal dominant HLA-linked spinocerebellar ataxia (SCA1) locus, in two kindreds, within an 8-cM subregion of chromosome 6p. Am J Hum Genet. 1991 Jul;49(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- Ranum L. P., Rich S. S., Nance M. A., Duvick L. A., Aita J. F., Orr H. T., Anton-Johnson S., Schut L. J. Autosomal dominant spinocerebellar ataxia: locus heterogeneity in a Nebraska kindred. Neurology. 1992 Feb;42(2):344–347. doi: 10.1212/wnl.42.2.344. [DOI] [PubMed] [Google Scholar]
- Sakai T., Ohta M., Ishino H. Joseph disease in a non-Portuguese family. Neurology. 1983 Jan;33(1):74–80. doi: 10.1212/wnl.33.1.74. [DOI] [PubMed] [Google Scholar]
- Snell R. G., MacMillan J. C., Cheadle J. P., Fenton I., Lazarou L. P., Davies P., MacDonald M. E., Gusella J. F., Harper P. S., Shaw D. J. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington's disease. Nat Genet. 1993 Aug;4(4):393–397. doi: 10.1038/ng0893-393. [DOI] [PubMed] [Google Scholar]
- Strong T. V., Tagle D. A., Valdes J. M., Elmer L. W., Boehm K., Swaroop M., Kaatz K. W., Collins F. S., Albin R. L. Widespread expression of the human and rat Huntington's disease gene in brain and nonneural tissues. Nat Genet. 1993 Nov;5(3):259–265. doi: 10.1038/ng1193-259. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Nishizawa M., Tanaka H., Kawashima S., Sakamoto H., Karube Y., Shimazaki H., Soutome M., Endo K., Ohta S. The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet. 1993 Jul;4(3):300–304. doi: 10.1038/ng0793-300. [DOI] [PubMed] [Google Scholar]
- Telenius H., Kremer H. P., Theilmann J., Andrew S. E., Almqvist E., Anvret M., Greenberg C., Greenberg J., Lucotte G., Squitieri F. Molecular analysis of juvenile Huntington disease: the major influence on (CAG)n repeat length is the sex of the affected parent. Hum Mol Genet. 1993 Oct;2(10):1535–1540. doi: 10.1093/hmg/2.10.1535. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Yakura H., Wakisaka A., Fujimoto S., Itakura K. Letter: Hereditary ataxia and HL-A. N Engl J Med. 1974 Jul 18;291(3):154–155. doi: 10.1056/NEJM197407182910314. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Jodice C., Sandkuijl L. A., Kwiatkowski T. J., Jr, McCall A. E., Huntoon S. A., Lulli P., Spadaro M., Litt M., Cann H. M. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps telomeric to the HLA complex and is closely linked to the D6S89 locus in three large kindreds. Am J Hum Genet. 1991 Jul;49(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Zoghbi H. Y., Pollack M. S., Lyons L. A., Ferrell R. E., Daiger S. P., Beaudet A. L. Spinocerebellar ataxia: variable age of onset and linkage to human leukocyte antigen in a large kindred. Ann Neurol. 1988 Jun;23(6):580–584. doi: 10.1002/ana.410230609. [DOI] [PubMed] [Google Scholar]
- Zühlke C., Riess O., Schröder K., Siedlaczck I., Epplen J. T., Engel W., Thies U. Expansion of the (CAG)n repeat causing Huntington's disease in 352 patients of German origin. Hum Mol Genet. 1993 Sep;2(9):1467–1469. doi: 10.1093/hmg/2.9.1467. [DOI] [PubMed] [Google Scholar]


