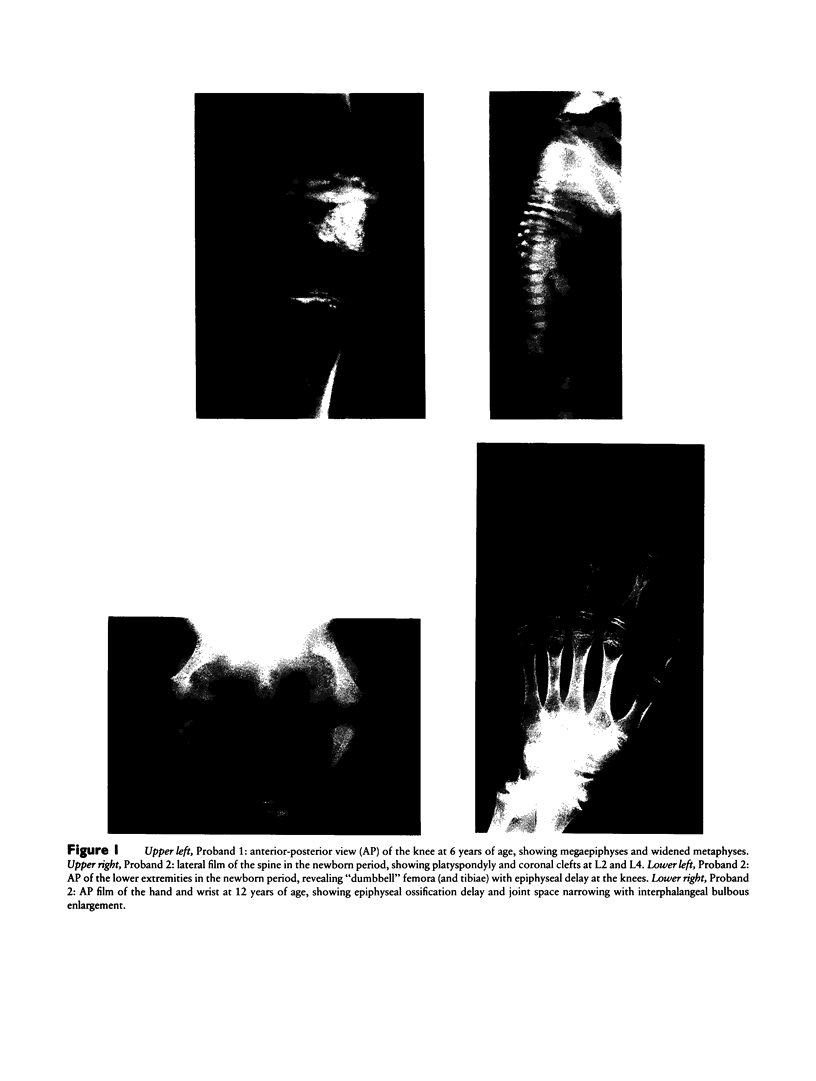
Abstract
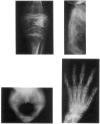
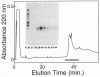
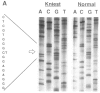
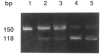
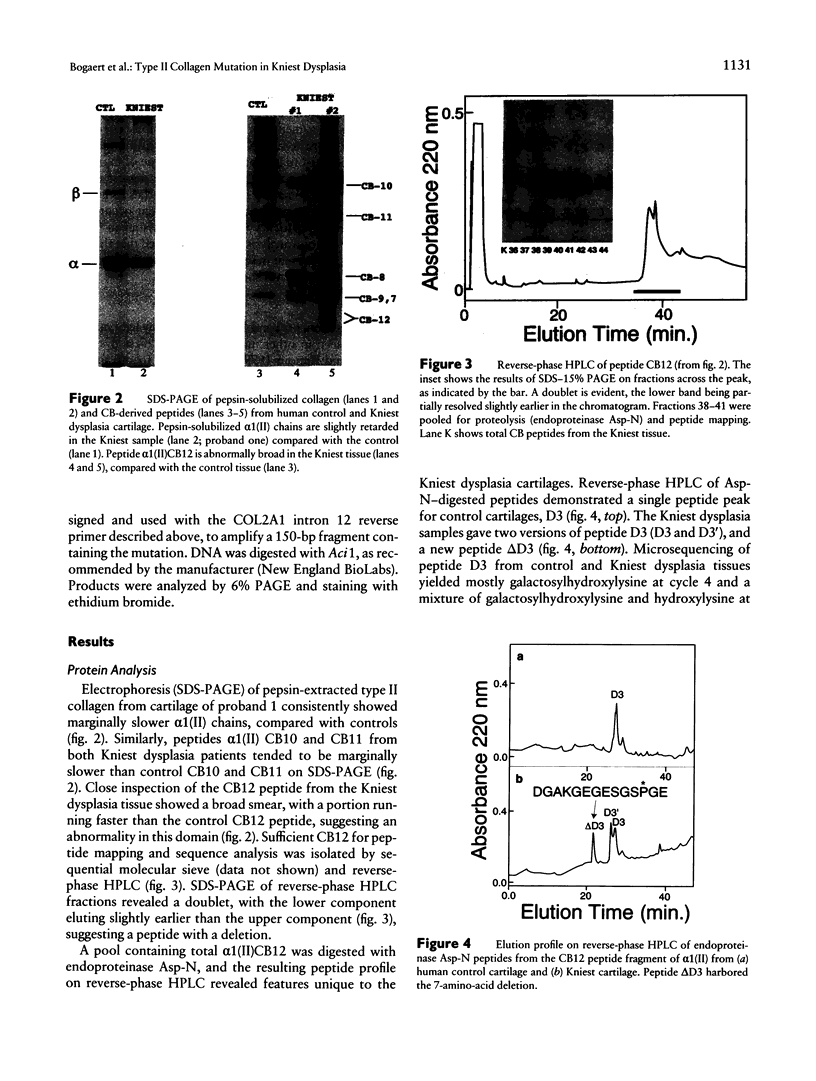
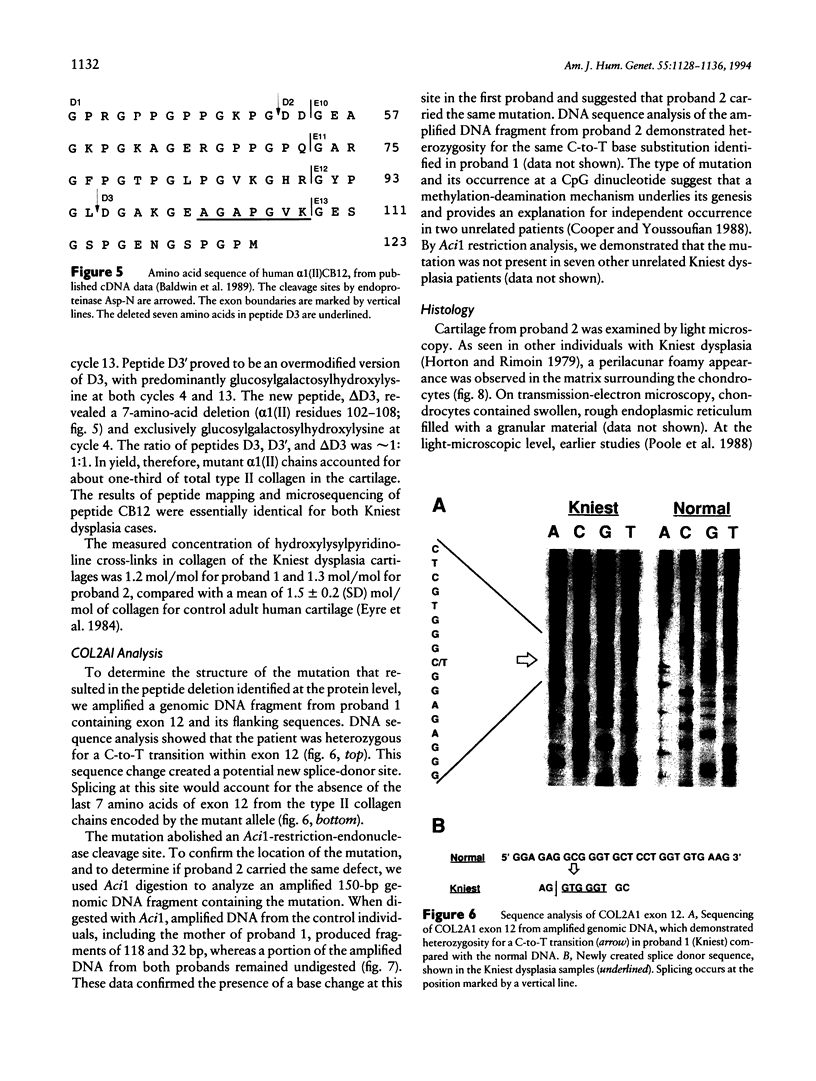
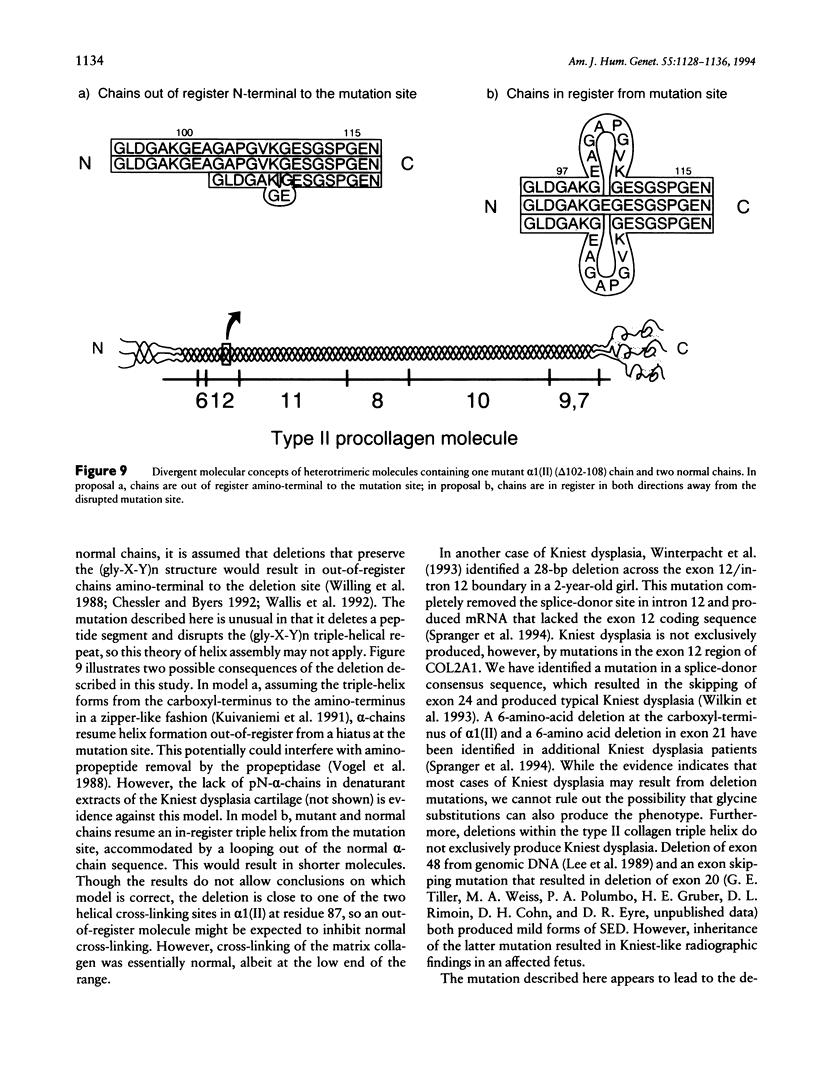
Kniest dysplasia is a heritable chondrodysplasia that severely affects skeletal growth. Recent evidence suggests that the etiology is based on mutations in COL2A1, the gene for collagen type II. We report the detection and partial characterization of an identical defect in type II collagen in two unrelated patients with Kniest dysplasia. Analysis of cyanogen bromide (CB)-digested cartilage samples from both probands by SDS-PAGE revealed an abnormal band for peptide alpha 1(II)CB12. The peptide was purified and digested with endoproteinase Asp-N. Fragments unique to the Kniest tissues were identified by reverse-phase high-pressure liquid chromatography and by sequence analysis. The results established a deletion of amino acids 102-108 of the alpha 1(II) triple-helical domain, which disrupted the (gly-X-Y)n repeat needed for helix formation. This was confirmed by sequence analysis of DNA amplified from both probands, revealing the molecular basis to be a single nucleotide mutation at a CpG dinucleotide (GCG-->GTG) in the codon for alanine 102. The mutation created a new splice donor site, which would account for the absence of the last seven amino acids from the 3' end of exon 12 in alpha 1(II)CB12. Light and electron micrographs of the probands' cartilage showed the perilacunar foamy matrix ("Swiss cheese") characteristic of Kniest dysplasia and chondrocytes containing dilated rough endoplasmic reticulum, which earlier studies had shown were filled with type II procollagen. These two cases strengthen the concept that Kniest dysplasia is based on mutations of COL2A1 and belongs within the broad spectrum of chondrodysplasias caused by type II collagenopathies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N. N., Ala-Kokko L., Knowlton R. G., Jimenez S. A., Weaver E. J., Maguire J. I., Tasman W., Prockop D. J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N. N., McDonald-McGinn D. M., Zackai E. H., Knowlton R. G., LaRossa D., DiMascio J., Prockop D. J. A second mutation in the type II procollagen gene (COL2AI) causing stickler syndrome (arthro-ophthalmopathy) is also a premature termination codon. Am J Hum Genet. 1993 Jan;52(1):39–45. [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Prockop D. J. Completion of the intron-exon structure of the gene for human type II procollagen (COL2A1): variations in the nucleotide sequences of the alleles from three chromosomes. Genomics. 1990 Nov;8(3):454–460. doi: 10.1016/0888-7543(90)90031-o. [DOI] [PubMed] [Google Scholar]
- Baldwin C. T., Reginato A. M., Smith C., Jimenez S. A., Prockop D. J. Structure of cDNA clones coding for human type II procollagen. The alpha 1(II) chain is more similar to the alpha 1(I) chain than two other alpha chains of fibrillar collagens. Biochem J. 1989 Sep 1;262(2):521–528. doi: 10.1042/bj2620521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Rapid fractionation of collagen chains and peptides by high-performance liquid chromatography. Anal Biochem. 1986 Apr;154(1):338–344. doi: 10.1016/0003-2697(86)90534-8. [DOI] [PubMed] [Google Scholar]
- Bogaert R., Tiller G. E., Weis M. A., Gruber H. E., Rimoin D. L., Cohn D. H., Eyre D. R. An amino acid substitution (Gly853-->Glu) in the collagen alpha 1(II) chain produces hypochondrogenesis. J Biol Chem. 1992 Nov 5;267(31):22522–22526. [PubMed] [Google Scholar]
- Chessler S. D., Byers P. H. Defective folding and stable association with protein disulfide isomerase/prolyl hydroxylase of type I procollagen with a deletion in the pro alpha 2(I) chain that preserves the Gly-X-Y repeat pattern. J Biol Chem. 1992 Apr 15;267(11):7751–7757. [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Koob T. J., Van Ness K. P. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984 Mar;137(2):380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. The distribution of different molecular species of collagen in fibrous, elastic and hyaline cartilages of the pig. Biochem J. 1975 Dec;151(3):595–602. doi: 10.1042/bj1510595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Upton M. P., Shapiro F. D., Wilkinson R. H., Vawter G. F. Nonexpression of cartilage type II collagen in a case of Langer-Saldino achondrogenesis. Am J Hum Genet. 1986 Jul;39(1):52–67. [PMC free article] [PubMed] [Google Scholar]
- Eyre D. Collagen cross-linking amino acids. Methods Enzymol. 1987;144:115–139. doi: 10.1016/0076-6879(87)44176-1. [DOI] [PubMed] [Google Scholar]
- Horton W. A., Machado M. A., Ellard J., Campbell D., Bartley J., Ramirez F., Vitale E., Lee B. Characterization of a type II collagen gene (COL2A1) mutation identified in cultured chondrocytes from human hypochondrogenesis. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4583–4587. doi: 10.1073/pnas.89.10.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton W. A., Rimoin D. L. Kniest dysplasia. A histochemical study of the growth plate. Pediatr Res. 1979 Nov;13(11):1266–1270. doi: 10.1203/00006450-197911000-00012. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Körkkö J., Ritvaniemi P., Haataja L., Käriäinen H., Kivirikko K. I., Prockop D. J., Ala-Kokko L. Mutation in type II procollagen (COL2A1) that substitutes aspartate for glycine alpha 1-67 and that causes cataracts and retinal detachment: evidence for molecular heterogeneity in the Wagner syndrome and the Stickler syndrome (arthro-ophthalmopathy) Am J Hum Genet. 1993 Jul;53(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Rosenberg L., Hollister D., Murray L., Rimoin D. Kniest dysplasia is characterized by an apparent abnormal processing of the C-propeptide of type II cartilage collagen resulting in imperfect fibril assembly. J Clin Invest. 1988 Feb;81(2):579–589. doi: 10.1172/JCI113356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEGEMANN H. Mikrobestimmung von Hydroxyprolin mit Chloramin-T und p-Dimethylaminobenzaldehyd. Hoppe Seylers Z Physiol Chem. 1958;311(1-3):41–45. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Spranger J., Winterpacht A., Zabel B. The type II collagenopathies: a spectrum of chondrodysplasias. Eur J Pediatr. 1994 Feb;153(2):56–65. doi: 10.1007/BF01959208. [DOI] [PubMed] [Google Scholar]
- Tiller G. E., Rimoin D. L., Murray L. W., Cohn D. H. Tandem duplication within a type II collagen gene (COL2A1) exon in an individual with spondyloepiphyseal dysplasia. Proc Natl Acad Sci U S A. 1990 May;87(10):3889–3893. doi: 10.1073/pnas.87.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikkula M., Ritvaniemi P., Vuorio A. F., Kaitila I., Ala-Kokko L., Peltonen L. A mutation in the amino-terminal end of the triple helix of type II collagen causing severe osteochondrodysplasia. Genomics. 1993 Apr;16(1):282–285. doi: 10.1006/geno.1993.1179. [DOI] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]
- Vogel B. E., Doelz R., Kadler K. E., Hojima Y., Engel J., Prockop D. J. A substitution of cysteine for glycine 748 of the alpha 1 chain produces a kink at this site in the procollagen I molecule and an altered N-proteinase cleavage site over 225 nm away. J Biol Chem. 1988 Dec 15;263(35):19249–19255. [PubMed] [Google Scholar]
- Wallis G. A., Kadler K. E., Starman B. J., Byers P. H. A tripeptide deletion in the triple-helical domain of the pro alpha 1(I) chain of type I procollagen in a patient with lethal osteogenesis imperfecta does not alter cleavage of the molecule by N-proteinase. J Biol Chem. 1992 Dec 15;267(35):25529–25534. [PubMed] [Google Scholar]
- Williams C. J., Considine E. L., Knowlton R. G., Reginato A., Neumann G., Harrison D., Buxton P., Jimenez S., Prockop D. J. Spondyloepiphyseal dysplasia and precocious osteoarthritis in a family with an Arg75-->Cys mutation in the procollagen type II gene (COL2A1). Hum Genet. 1993 Nov;92(5):499–505. doi: 10.1007/BF00216458. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Starman B., Holbrook K. A., Greenberg C. R., Byers P. H. Heterozygosity for a large deletion in the alpha 2(I) collagen gene has a dramatic effect on type I collagen secretion and produces perinatal lethal osteogenesis imperfecta. J Biol Chem. 1988 Jun 15;263(17):8398–8404. [PubMed] [Google Scholar]
- Winterpacht A., Hilbert M., Schwarze U., Mundlos S., Spranger J., Zabel B. U. Kniest and Stickler dysplasia phenotypes caused by collagen type II gene (COL2A1) defect. Nat Genet. 1993 Apr;3(4):323–326. doi: 10.1038/ng0493-323. [DOI] [PubMed] [Google Scholar]