Abstract
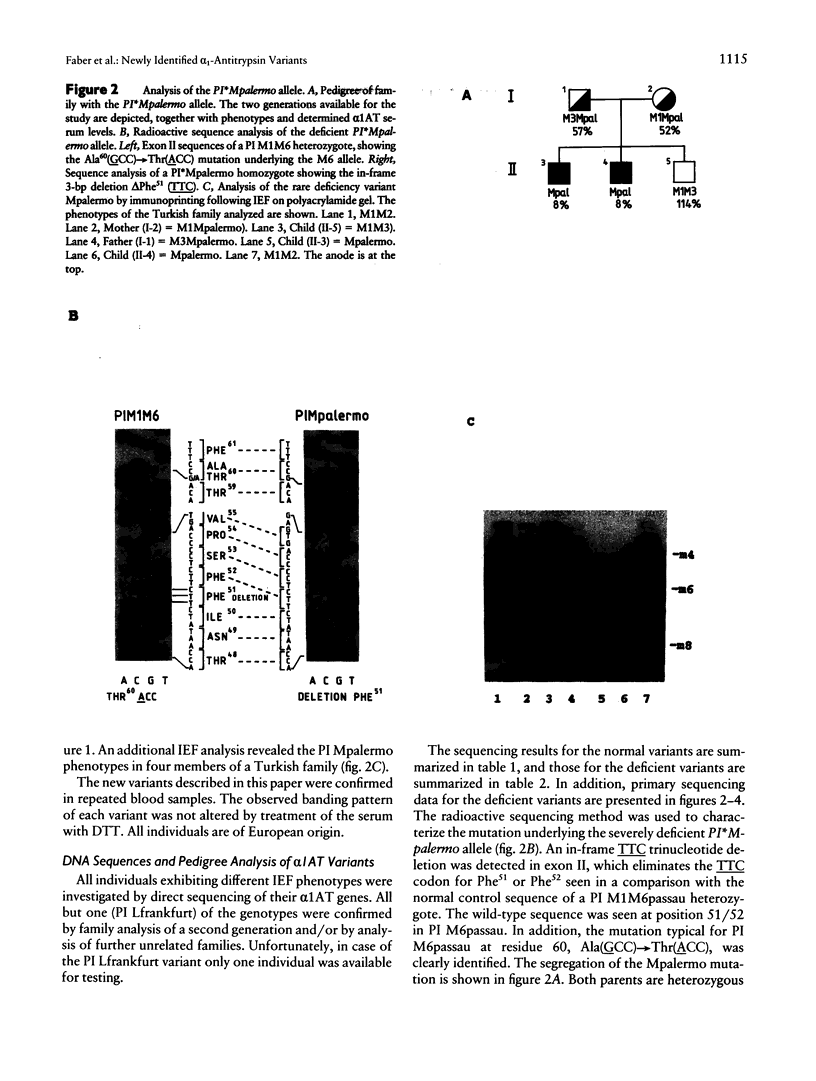
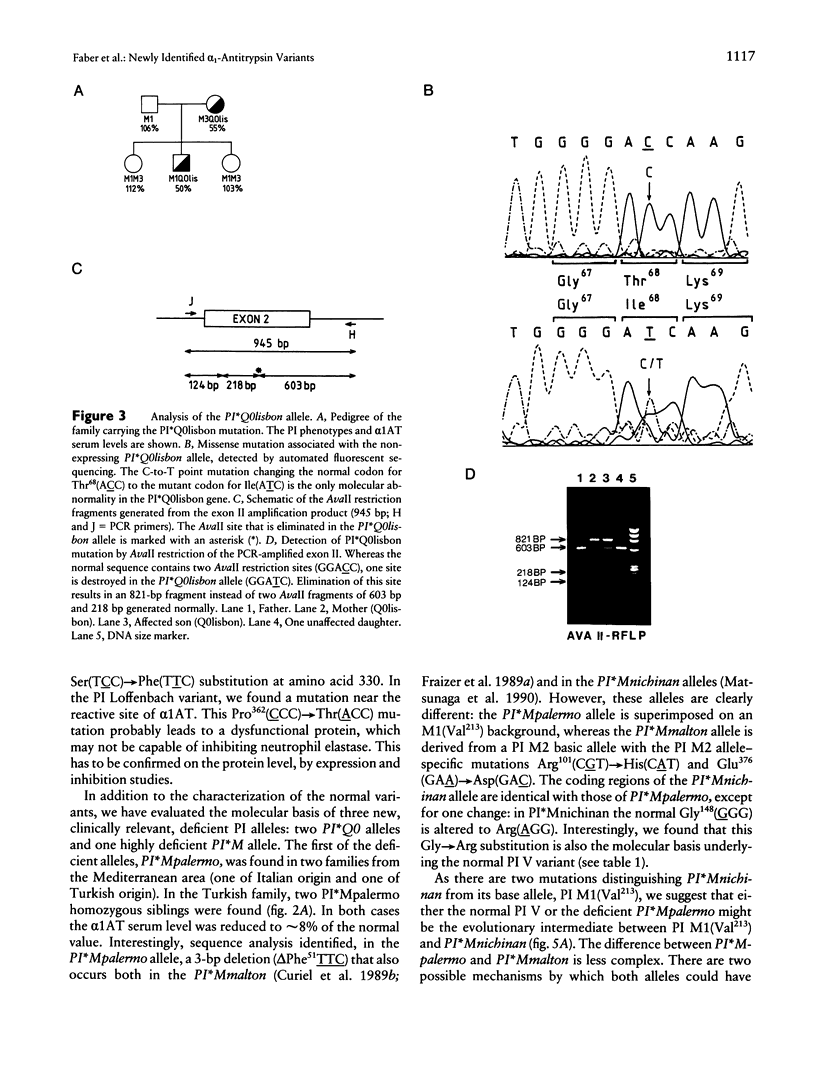
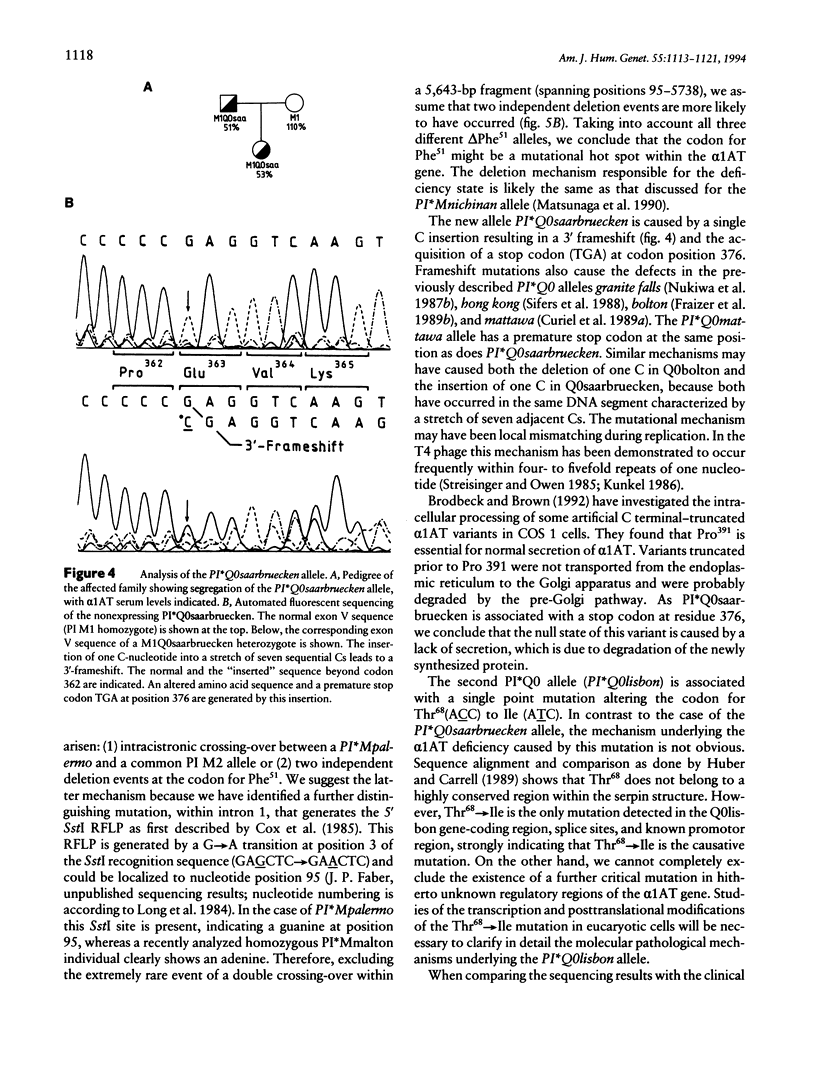
We have investigated the molecular basis of 15 new alpha 1-antitrypsin (alpha 1AT) variants. Phenotyping by isoelectric focusing (IEF) was used as a screening method to detect alpha 1AT variants at the protein level. Genotyping was then performed by sequence analysis of all coding exons, exon-intron junctions, and the hepatocyte-specific promoter region including exon Ic. Three of these rare variants are alleles of clinical relevance, associated with undetectable or very low serum levels of alpha 1AT:the PI*Q0saarbruecken allele generated by a 1-bp C-nucleotide insertion within a stretch of seven cytosines spanning residues 360-362, resulting in a 3' frameshift and the acquisition of a stop codon at residue 376; a point mutation in the PI*Q0lisbon allele, resulting in a single amino acid substitution Thr68(ACC)-->Ile(ATC); and an in-frame trinucleotide deletion delta Phe51 (TTC) in the highly deficient PI*Mpalermo allele. The remaining 12 alleles are associated with normal alpha 1AT serum levels and are characterized by point mutations causing single amino acid substitutions in all but one case. This exception is a silent mutation, which does not affect the amino acid sequence. The limitation of IEF compared with DNA sequence analysis, for identification of new variants, their generation by mutagenesis, and the clinical relevance of the three deficiency alleles are discussed.
Full text
PDF


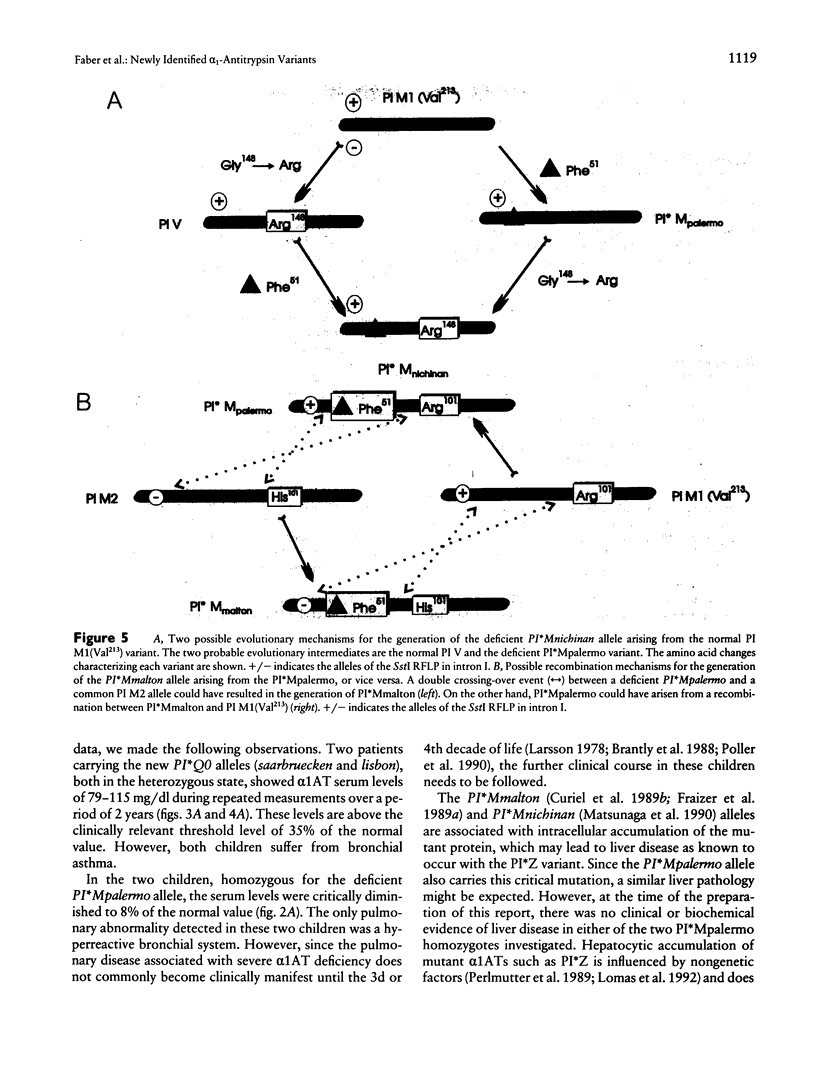


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brantly M. L., Paul L. D., Miller B. H., Falk R. T., Wu M., Crystal R. G. Clinical features and history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with pulmonary symptoms. Am Rev Respir Dis. 1988 Aug;138(2):327–336. doi: 10.1164/ajrccm/138.2.327. [DOI] [PubMed] [Google Scholar]
- Brodbeck R. M., Brown J. L. Secretion of alpha-1-proteinase inhibitor requires an almost full length molecule. J Biol Chem. 1992 Jan 5;267(1):294–297. [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Woo S. L., Mansfield T. DNA restriction fragments associated with alpha 1-antitrypsin indicate a single origin for deficiency allele PI Z. Nature. 1985 Jul 4;316(6023):79–81. doi: 10.1038/316079a0. [DOI] [PubMed] [Google Scholar]
- Crystal R. G. Alpha 1-antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J Clin Invest. 1990 May;85(5):1343–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D. T., Holmes M. D., Okayama H., Brantly M. L., Vogelmeier C., Travis W. D., Stier L. E., Perks W. H., Crystal R. G. Molecular basis of the liver and lung disease associated with the alpha 1-antitrypsin deficiency allele Mmalton. J Biol Chem. 1989 Aug 15;264(23):13938–13945. [PubMed] [Google Scholar]
- Curiel D., Brantly M., Curiel E., Stier L., Crystal R. G. Alpha 1-antitrypsin deficiency caused by the alpha 1-antitrypsin Nullmattawa gene. An insertion mutation rendering the alpha 1-antitrypsin gene incapable of producing alpha 1-antitrypsin. J Clin Invest. 1989 Apr;83(4):1144–1152. doi: 10.1172/JCI113994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSSON S. PULMONARY EMPHYSEMA AND ALPHA1-ANTITRYPSIN DEFICIENCY. Acta Med Scand. 1964 Feb;175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Faber J. P., Poller W., Olek K., Baumann U., Carlson J., Lindmark B., Eriksson S. The molecular basis of alpha 1-antichymotrypsin deficiency in a heterozygote with liver and lung disease. J Hepatol. 1993 Jul;18(3):313–321. doi: 10.1016/s0168-8278(05)80275-2. [DOI] [PubMed] [Google Scholar]
- Faber J. P., Weidinger S., Goedde H. W., Ole K. The deficient alpha-I-antitrypsin phenotype PI P is associated with an A-to-T transversion in exon III of the gene. Am J Hum Genet. 1989 Jul;45(1):161–163. [PMC free article] [PubMed] [Google Scholar]
- Faber J. P., Weidinger S., Olek K. Sequence data of the rare deficient alpha 1-antitrypsin variant PI Zaugsburg. Am J Hum Genet. 1990 Jun;46(6):1158–1162. [PMC free article] [PubMed] [Google Scholar]
- Fraizer G. C., Harrold T. R., Hofker M. H., Cox D. W. In-frame single codon deletion in the Mmalton deficiency allele of alpha 1-antitrypsin. Am J Hum Genet. 1989 Jun;44(6):894–902. [PMC free article] [PubMed] [Google Scholar]
- Fraizer G. C., Siewertsen M., Harrold T. R., Cox D. W. Deletion/frameshift mutation in the alpha 1-antitrypsin null allele, PI*QObolton. Hum Genet. 1989 Nov;83(4):377–382. doi: 10.1007/BF00291385. [DOI] [PubMed] [Google Scholar]
- Holmes M. D., Brantly M. L., Crystal R. G. Molecular analysis of the heterogeneity among the P-family of alpha-1-antitrypsin alleles. Am Rev Respir Dis. 1990 Nov;142(5):1185–1192. doi: 10.1164/ajrccm/142.5.1185. [DOI] [PubMed] [Google Scholar]
- Huber R., Carrell R. W. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989 Nov 14;28(23):8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Frameshift mutagenesis by eucaryotic DNA polymerases in vitro. J Biol Chem. 1986 Oct 15;261(29):13581–13587. [PubMed] [Google Scholar]
- Kühnl P., Spielmann W. PiT: a new allele in the alpha 1-antitrypsin system. Hum Genet. 1979;50(2):221–223. doi: 10.1007/BF00390245. [DOI] [PubMed] [Google Scholar]
- Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med Scand. 1978;204(5):345–351. doi: 10.1111/j.0954-6820.1978.tb08452.x. [DOI] [PubMed] [Google Scholar]
- Lomas D. A., Evans D. L., Finch J. T., Carrell R. W. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992 Jun 18;357(6379):605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Matsunaga E., Shiokawa S., Nakamura H., Maruyama T., Tsuda K., Fukumaki Y. Molecular analysis of the gene of the alpha 1-antitrypsin deficiency variant, Mnichinan. Am J Hum Genet. 1990 Mar;46(3):602–612. [PMC free article] [PubMed] [Google Scholar]
- Nukiwa T., Brantly M., Ogushi F., Fells G., Satoh K., Stier L., Courtney M., Crystal R. G. Characterization of the M1(Ala213) type of alpha 1-antitrypsin, a newly recognized, common "normal" alpha 1-antitrypsin haplotype. Biochemistry. 1987 Aug 25;26(17):5259–5267. doi: 10.1021/bi00391a008. [DOI] [PubMed] [Google Scholar]
- Nukiwa T., Takahashi H., Brantly M., Courtney M., Crystal R. G. alpha 1-Antitrypsin nullGranite Falls, a nonexpressing alpha 1-antitrypsin gene associated with a frameshift to stop mutation in a coding exon. J Biol Chem. 1987 Sep 5;262(25):11999–12004. [PubMed] [Google Scholar]
- Perlino E., Cortese R., Ciliberto G. The human alpha 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J. 1987 Sep;6(9):2767–2771. doi: 10.1002/j.1460-2075.1987.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Kay R. M., Cole F. S., Rossing T. H., Van Thiel D., Colten H. R. The cellular defect in alpha 1-proteinase inhibitor (alpha 1-PI) deficiency is expressed in human monocytes and in Xenopus oocytes injected with human liver mRNA. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6918–6921. doi: 10.1073/pnas.82.20.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Schlesinger M. J., Pierce J. A., Punsal P. I., Schwartz A. L. Synthesis of stress proteins is increased in individuals with homozygous PiZZ alpha 1-antitrypsin deficiency and liver disease. J Clin Invest. 1989 Nov;84(5):1555–1561. doi: 10.1172/JCI114332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poller W., Faber J. P., Olek K. Highly variable clinical course in severe alpha 1-antitrypsin deficiency--use of polymerase chain reaction for the detection of rare deficiency alleles. Klin Wochenschr. 1990 Sep 3;68(17):857–863. doi: 10.1007/BF01662782. [DOI] [PubMed] [Google Scholar]
- Poller W., Faber J. P., Weidinger S., Olek K. DNA polymorphisms associated with a new alpha 1-antitrypsin PIQ0 variant (PIQ0riedenburg). Hum Genet. 1991 Mar;86(5):522–524. doi: 10.1007/BF00194647. [DOI] [PubMed] [Google Scholar]
- Salmon J. E., Edberg J. C., Brogle N. L., Kimberly R. P. Allelic polymorphisms of human Fc gamma receptor IIA and Fc gamma receptor IIIB. Independent mechanisms for differences in human phagocyte function. J Clin Invest. 1992 Apr;89(4):1274–1281. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifers R. N., Brashears-Macatee S., Kidd V. J., Muensch H., Woo S. L. A frameshift mutation results in a truncated alpha 1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem. 1988 May 25;263(15):7330–7335. [PubMed] [Google Scholar]
- Streisinger G., Owen J. Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics. 1985 Apr;109(4):633–659. doi: 10.1093/genetics/109.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger S. Reliable phenotyping of alpha-1-antitrypsin by hybrid isoelectric focusing in an ultranarrow immobilized pH gradient. Electrophoresis. 1992 Apr;13(4):234–239. doi: 10.1002/elps.1150130148. [DOI] [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Crystal R. G. Comparison of alpha-1-antitrypsin levels and antineutrophil elastase capacity of blood and lung in a patient with the alpha-1-antitrypsin phenotype null-null before and during alpha-1-antitrypsin augmentation therapy. Am Rev Respir Dis. 1987 Mar;135(3):539–543. doi: 10.1164/arrd.1987.135.3.539. [DOI] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]