Abstract
Bat echolocation calls provide remarkable examples of ‘good design’ through evolution by natural selection. Theory developed from acoustics and sonar engineering permits a strong predictive basis for understanding echolocation performance. Call features, such as frequency, bandwidth, duration and pulse interval are all related to ecological niche. Recent technological breakthroughs have aided our understanding of adaptive aspects of call design in free-living bats. Stereo videogrammetry, laser scanning of habitat features and acoustic flight path tracking permit reconstruction of the flight paths of echolocating bats relative to obstacles and prey in nature. These methods show that echolocation calls are among the most intense airborne vocalizations produced by animals. Acoustic tracking has clarified how and why bats vary call structure in relation to flight speed. Bats using broadband echolocation calls adjust call design in a range-dependent manner so that nearby obstacles are localized accurately. Recent phylogenetic analyses based on gene sequences show that particular types of echolocation signals have evolved independently in several lineages of bats. Call design is often influenced more by perceptual challenges imposed by the environment than by phylogeny, and provides excellent examples of convergent evolution. Now that whole genome sequences of bats are imminent, understanding the functional genomics of echolocation will become a major challenge.
Keywords: echolocation, bats, adaptation, convergent evolution
1. Introduction
In ‘The Blind Watchmaker’, Dawkins (1986) uses bat echolocation to illustrate features of ‘good design’ through evolution by natural selection. Since the design of the echolocation calls determines the type and quality of information contained in returning echoes, bats have evolved different signals to meet a diversity of sensory demands. Dawkins poses problems that echolocating bats experience, considers solutions that sensible engineers might consider and then arrives at solutions that bats have achieved. Often the solutions adopted by bats, for example, the use of broadband chirps for ranging, and the exploitation of Doppler shifts to calculate relative velocity, have parallels with methods that engineers have adopted in commercial and military uses of sonar and radar. Bat echolocation provides rich examples of good design because echolocation performance can be predicted from theory developed in acoustics and in sonar (and radar) engineering. For example, Rayleigh scattering calculations have been used to predict the sizes of insects detectable by different frequencies of sound used by echolocating bats (Houston et al. 2004). Ambiguity analysis used by radar engineers (Woodward 1953; Kelly & Wishner 1965) is useful in determining the Doppler tolerance and range resolution of signals (Altes & Titlebaum 1970; Boonman et al. 2003).
In this paper, we will show how recent advances in technology have allowed new insights into adaptive design in bat echolocation. We focus on field studies. We discuss how call features, such as frequency, intensity, duration and intervals between pulses are all shaped by the perceptual challenges faced by the bat in its natural environment. Specifically, we will show how bats using broadband signals adjust calls in relation to their flight speed so that objects can be localized with accuracy. We will argue that signal design in bats provides remarkable examples of convergent evolution, with distantly related taxa evolving similar signal designs independently in the face of similar environmental challenges. We will conclude by anticipating how recent advances in genomics might increase our understanding of the genetic basis for the evolution of echolocation. We suggest that engineers can learn from breakthroughs in our understanding of adaptive design in echolocation signals used by bats. For example, autonomously guided vehicles could use speed-dependent sonar signal designs that minimize localization errors, offering advances over the simple signals used in many current robotics applications (e.g. Gao & Hinders 2006).
2. Methodological advances
To understand how bats use echolocation in the field, it is necessary to quantify the bat's position in relation to surrounding obstacles and insect prey. Signal design can then be related to the proximity and nature of obstacles, and changes in signal structure can be monitored as targets are approached. Biologists have used multiflash photography to reconstruct the flight paths of wild bats in three dimensions for some time using stereo photogrammetry (e.g. Jones & Rayner 1988; Kalko & Schnitzler 1993). More recently, two infrared video cameras have been used in conjunction with measuring microphones. Since the bat's position can be quantified accurately in three dimensions, its distance and position relative to a calibrated microphone can be reconstructed, thus allowing investigation of changes in call design and intensity during flight (Holderied et al. 2005). Flight paths can also be reconstructed in three dimensions using acoustic, rather than optical methods. Time-of-arrival differences of calls recorded at microphone arrays allow the bat's position at the time of calling to be measured accurately (e.g. Holderied & von Helversen 2003). Bats couple call production with wing flapping to minimize the costs of echolocation during flight (Speakman & Racey 1991). Bats flap and call at high rates in flight (often 5–20 Hz), thus allowing frequent updates of positional data recorded at microphone arrays, which string together in a pearl-chain-like manner to trace the bat's flight trajectory. Holderied & von Helversen (2003) used two arrays of four microphones, with each arranged in a symmetrical star, to reconstruct flight paths accurately, with a direction-dependent location accuracy of between 0.2% and 2% of the distance (Aubauer 1994). Depending on the species under study, the tracking range is up to 35 m.
3. Frequency
The range of frequencies exploited by echolocating bats makes perfect sense from an acoustics perspective. Bat echolocation calls vary in their dominant frequency approximately between 11 kHz (e.g. Euderma maculatum; Fullard & Dawson 1997) and 212 kHz (Cloeotis percivali; Fenton & Bell 1981). Most insectivorous bats call with dominant frequencies between 20 kHz and 60 kHz (Fenton et al. 1998). Lower frequencies are avoided because echoes from insect-sized targets are weak when the wavelength is longer than the insect wing length (Houston et al. 2004). For example, target strength (the ratio between incident and echo sound pressure) is reduced by approximately 25 dB at 1 m when the ratio of target wing length/sound wavelength drops from 1 to 0.2 (Houston et al. 2004). High frequencies are therefore necessary to detect small targets such as aerial insects. However, atmospheric attenuation is frequency-dependent, and limits the effective range of echolocation at high frequencies (Lawrence & Simmons 1982).
Call frequency in closely related bat species may also diverge to facilitate intraspecific communication, rather than to facilitate resource partitioning by allowing the species to forage more effectively on different size classes of prey related to call wavelength differences. Cryptic species of bats are morphologically similar in appearance, but often differ in echolocation call frequency (Jones & Barlow 2004). The wavelength differences between species are, however, too small to influence differences in target strengths from insects within the size range of the prey taken (Jones & Barlow 2004), and acoustic divergence probably evolved instead so that each species has its own ‘private bandwidth’ with which it can communicate effectively with conspecifics (Heller & von Helversen 1989; Kingston et al. 2000; Thabah et al. 2006). Some species of horseshoe bats have nevertheless changed call frequency radically during evolution using different harmonics in a harmonic series of frequencies (Kingston & Rossiter 2004). Such ‘harmonic hopping’ may allow bats to exploit different sizes of insect prey and may provide a mechanism for sympatric speciation (Kingston & Rossiter 2004).
More recently, attention on the adaptive significance of call frequency in bats has focused on the importance of call bandwidth. Bat species that emit calls with broad bandwidths are most successful in capturing insect prey close to clutter (clutter is defined as echoes other than the target of interest; Siemers & Schnitzler 2004). For example, the calls of Myotis nattereri sweep from 135 kHz to 16 kHz and hence span wavelengths from approximately 22 mm to 2.6 mm. Such calls will ensonify many reflecting surfaces, including prey and vegetation objects, simultaneously, thus allowing the bat to develop a detailed acoustic snapshot that allows it to separate prey from background clutter (Siemers & Schnitzler 2004).
4. Call intensity
Results from acoustic tracking and stereo videogrammetry have given measurements of the intensities of calls emitted by a range of echolocating bat species in the wild (Jensen & Miller 1999; Holderied & von Helversen 2003; Holderied et al. 2005). Call intensities greater than 125 dB peak equivalent sound pressure level (peSPL) at 10 cm have been recorded from aerial feeding species searching for insects, and measurements of 133 dB peSPL at 10 cm have been measured for species that fly fast in open spaces (Holderied & von Helversen 2003). Such values are among the highest intensities documented for airborne vocalizations by any animal. Interestingly, the maximum sound pressure levels of all aerial feeding species studied so far are very similar. Whereas the largest species weighing 80 g can reach 133 dB, even a bat species weighing only 4 g can reach 128 dB. Other species that forage in flight, but listen for walking noises of prey on the ground, such as Hemprich's long-eared bats Otonycteris hemprichii foraging on scorpions, have source levels of no more than 93 dB peSPL (average 82 dB; M. W. Holderied & C. Corine, unpublished results), which is why such bats have been called whispering bats. In this manner they reduce the masking effect of their calls while listening for the faint rustling noises of their prey. Understanding call intensity has played an important role in calculating potential detection ranges for insect targets, and this has helped in the refinement of hypotheses about how bats time the emission of calls (see §5).
5. Duration and pulse interval
Bats that call at low duty cycles (i.e. the proportion of time that the signal is ‘on’ is low) reduce call duration as they approach targets because they cannot tolerate overlap between the outgoing signal and the returning echo. Intense vocalizations mask any faint echo that returns during or soon after call emission. Moreover, some bat species contract muscles in their middle ear to avoid deafening themselves while calling (Suga & Jen 1975), and also employ neural attenuation mechanisms (Suga & Schlegel 1972) that make the detection of faint echoes impossible. The zone around the bat in which target echoes overlap with the emitted call is termed the signal overlap zone (SOZ; Kalko & Schnitzler 1993). Since sound travels at 340 m s−1 and because the bat has to wait for sound to reach and return from a target, each 1 ms of signal adds 17 cm to the SOZ. Bats therefore typically reduce call duration as they approach targets so that the SOZ is equal to or less than the distance to the target, and hence overlap between pulse and echo (forward masking) is avoided. Nevertheless, some overlap may exist during the final phases of prey capture (e.g. Britton et al. 1997) or in species that use exceptionally broad bandwidth calls close to clutter (Siemers & Schnitzler 2000).
If echoes from background clutter arrive soon after prey echoes, and interfere with neural activity evoked by the prey echoes, then backward masking may occur (Kalko & Schnitzler 1993). Bats are therefore predicted to use signal durations that result in echoes returning in an overlap-free window, in which both forward and backward maskings are avoided (Kalko & Schnitzler 1993).
The interval between pulses (pulse interval) is also shaped by natural selection so that echolocation is efficient. This can be shown for two different situations. First, during insect pursuit, bats produce the next call immediately after hearing the echo from the prey. Because echo delay decreases while the bat approaches its prey, the bat calls at ever shorter intervals. Just before capture, this ends in a characteristic rapid ‘feeding buzz’ with up to approximately 200 pulses produced per second (e.g. Kalko & Schnitzler 1998).
Second, bats flying in open spaces not only use longer pulses than those flying in clutter, but they also have longer intervals between pulses, presumably to grant that all echoes arriving from the preceding call will be received before the emission of the next call. This would be adaptive because late echoes from the first pulse, which arrive after the following pulse, might cause confusion. Potential errors in call–echo assignation either mean that distant objects are perceived at close range, potentially triggering unnecessary evasive manoeuvres, or, in the more relevant case, that obstacles might erroneously be perceived at a safe distance, while actually they are dangerously close. Indeed, calculated maximum detection ranges (which depend on assumptions regarding the bat's echo detection thresholds) are linked to preferred pulse intervals: during search phase, i.e. when no prey target has been detected yet, all vespertilionid bats studied so far produce their next call only when even echoes from flying objects at the outer limit of their echolocation range would have returned to them (Holderied & von Helversen 2003; Holderied et al. 2005). In this manner they maximize calling rate without risking call–echo assignation problems. This is particularly intriguing, as bats also couple their call emission to their wing beat cycle in order to greatly reduce energy expenditure for call production (Speakman & Racey 1991). Bats that fly in open spaces achieve optimal timing of their pulse intervals as well as energetically cheap call production by matching their detection range for flying objects with their wing beat period (Holderied & von Helversen 2003; Holderied et al. 2005).
In addition, certain large structures, such as walls or rock faces, can be detected at a considerably longer distance. To achieve correct call–echo assignation also in this case, bats flying fast in open spaces sometimes skip calling for one or more wing beats, seemingly to await such late echoes from distant targets (Holderied & von Helversen 2003). However, there rests one major disadvantage in waiting for the return of all echoes: for bats with long-range echolocation, pulse intervals get very long, such that the acoustic image is updated only at an unfavourably low rate. Neotropical emballonurid bats, like other species such as the noctule, found a solution for this problem—they can call more frequently because they alternate between different call types (Kingston et al. 2003). In that way, even echoes arriving after the following call can be correctly assigned, because all echoes carry their respective call's spectral signature (Jung et al. in press).
6. Call ‘shape’ and the distance of focus
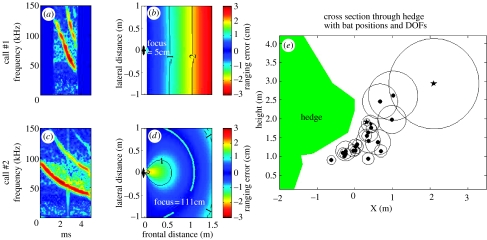
Besides the following of general rules, such as long narrowband calls for long-range detection in contrast to short broadband calls for accurate localization and extraction of object features (e.g. Schnitzler et al. 2003), bats show a remarkable range of call designs (figure 1) that change in a gradual manner. We are only beginning to understand and quantify the instantaneous adaptive value of such slight changes in call bandwidth, let alone in the curvature of a call during its frequency modulation. One new and promising approach builds on the fact that echolocation in flight bears two inevitable sources of error. The so-called ‘distance of focus’ (DOF) theory interprets the slight changes in signal design as a way to control flight-induced errors in distance measurements (Boonman et al. 2003; Holderied et al. 2006). First, in-flight ranging accuracy is disturbed because bats call at one place and hear the echo at a slightly different position along their flight trajectory. Second, all echoes will be affected by Doppler shifts, i.e. they are compressed in time and their pitch increases, which also perturbs distance measurements considerably. These two ranging errors together have a very interesting spatial distribution relative to the flying bat; the overall flight-induced ranging error will be 0 for all objects on one particular hemisphere with the bat in its centre. The farther the objects are from this hemisphere, the larger will be the flight-induced ranging error. Under natural conditions, such ranging errors can reach several centimetres. Thus, these errors may significantly increase collision risk in close-range navigation and impede capture success. What makes DOF theory so interesting is that every call design has its specific hemisphere radius, which depends on sweep rate and curvature (Boonman et al. 2003). By adjusting call design, bats can actively increase and decrease the radius of the hemisphere. It would be clearly adaptive to match the hemisphere to their target of interest in order to minimize flight-induced ranging errors. The name ‘distance of focus’ for the hemisphere radius has been chosen because this mechanism has similarities to focusing (i.e. accommodation) in vision.
Figure 1.
(a,c) Two calls of whiskered bats and (b,d) their respective horizontal flight-induced ranging error distributions. Zero ranging error is marked by a solid line labelled ‘focus’. a: Call no.1 is short and very broadband. b: This call design has a distance of focus (DOF) of 5 cm. c: Call no.2 is long and relatively narrowband. d: It has a DOF of 111 cm. Intermediate call designs have intermediate DOFs. e: Cross-section perpendicular through a flight corridor that follows a hedge. Symbols: Twenty-two individual bats' positions with respect to the hedge. Stars indicate bats using calls in a and c, respectively. Circles indicate DOF for the calls emitted closest to the cross-sectional plain (modified from Holderied et al. 2006).
In a field study with whiskered bats commuting in a narrow flight corridor along the surface of a hedgerow, Holderied et al. (2006) found distance-dependent signal design that supports the DOF theory: (i) DOFs were in a reasonable range from 1 cm to 110 cm, (ii) DOFs were shorter in more confined situations, and (iii) DOF in the confined situation was adjusted to the instantaneous distance to the hedge in a distance-dependent manner. Further studies will reveal whether other bat species in different ecological situations make similar use of plasticity in signal design.
7. Phylogenetic context
How bat echolocation calls are shaped by environmental challenges reveals exquisite examples of adaptation, and raises the question whether distantly related bat species that forage in similar habitats have independently evolved similar call designs to solve similar perceptual challenges. Phylogenetic analyses of genetic sequence data have revolutionized our understanding of evolutionary relationships among bat taxa. On the basis of morphology and echolocation characteristics, bats were previously classified into two suborders, the Megachiroptera (one family, the Old World fruit bats Pteropodidae) and the Microchiroptera (remaining 17 families; Gunnel & Simmons 2005). Only microchiropteran bats use laryngeal echolocation (calls produced in the larynx) and produce tonal signals in echolocation. No megachiropteran bats are known to echolocate, with the exception of bats in the genus Rousettus, which use tongue-clicking for orientation in caves. Traditionally, the brief clicks emitted by Rousettus were believed to offer poor orientation performance, yet the bats can perform as well as some microchiropteran bats in obstacle negotiation tasks. It has recently been proposed that the clicks emitted by Rousettus resemble Gabor functions, and hence minimize bandwidths at very short durations, focusing energy at frequencies that the bats hear best (Holland et al. 2004). Although clicks are also used by all other echolocating animals (toothed whales, swiftlets, oilbirds and insectivores), it is unlikely that they represent an ancestral state in the evolution of bat echolocation—it is more likely that echolocation evolved secondarily in bats in the genus Rousettus so that the bats can orient in dark caves (Springer et al. 2001; Jones & Teeling 2006).
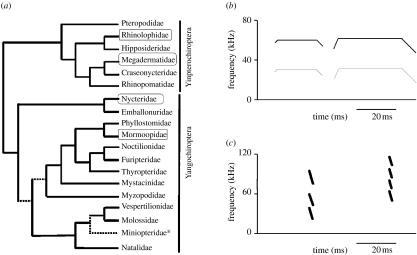
New molecular phylogenies based on large concatenated datasets from a series of nuclear and mitochondrial genes support the hypothesis that the Microchiroptera are paraphyletic (Teeling et al. 2000, 2005; Springer et al. 2001). Microbat paraphyly is supported by DNA–DNA hybridization (Hutcheon & Kirsch 2004), rare genomic events (Teeling et al. 2000, 2005), and by examination of extensive nuclear (Eick et al. 2005) and mitochondrial (Van Den Bussche & Hoofer 2004) datasets. In the new molecular phylogenies (figure 2), a deep divergence occurs between the Pteropodidae, Rhinolophidae, Hipposideridae, Megadermatidae, Craseonycteridae and Rhinopomatidae (now placed in the putative suborder Yinpterochiroptera), and the remaining 12 families of bats classified in the redefined Yangochiroptera. The implications of microbat paraphyly are profound; horseshoe bats (family Rhinolophidae) have the most sophisticated echolocation of any animals. They combine broadband and constant frequency components in calls that allow excellent performance for the key tasks of detection, localization and classification of targets (Schnitzler et al. 2003). The constant frequency facilitates detection (Schnitzler et al. 2003) and classification (von der Emde & Schnitzler 1990) of insect prey by facilitating the perception of ‘glints’ (small changes in the amplitude and frequency content of echoes caused by insect wing beats), while a terminal broadband sweep improves the localization of targets (Tian & Schnitzler 1997). However, horseshoe bats are in a clade that is sister to the Pteropodidae, bats which do not use laryngeal echolocation. Studies that use a molecular scaffold alongside morphological analyses of fossil data support the hypothesis that echolocation was present in the common ancestor of all bats, and was lost in the Pteropodidae only to evolve secondarily by tongue-clicking in cave-dwelling bats in the genus Rousettus (Springer et al. 2001). In contrast, one recent study has suggested that echolocation may have evolved twice in bats, once in the common ancestor of the Yangochiroptera, and again in the Yinpterochiroptera in the common ancestor of bats in all families in this clade that excludes the Pteropodidae (Eick et al. 2005). Resolution of the debate about how many times echolocation evolved in bats remains an exciting challenge.
Figure 2.
(a) The new molecular phylogeny of bat families. Associations that were not strongly supported by at least one independent molecular study are indicated by hatched lines. The position of the newly proposed family Miniopteridae is indicated by an asterisk. The tree unites the pteropodids, which do not use laryngeal echolocation, with the echolocating superfamily Rhinolophoidea (e.g. horseshoe bats) in the clade Yinpterochiroptera. All other bats that use laryngeal echolocation are united in the clade Yangochiroptera. Modified from Jones & Teeling (2006). (b,c) Some examples of convergent evolution in signal design. In (b), schematics of signals from Pteronotus parnellii (Yangochiroptera: Mormoopidae; left call) and Rhinolophus pearsonii (Yinpterochiroptera: Rhinolophidae; right call) are shown alongside one another. Both species emit long, constant frequency calls of similar frequency, with the constant frequency portion initiated and terminated by brief broadband sweeps. In both the cases, most energy is in the second harmonic of the call. In (c), a call of Nycteris thebaica (Yangochiroptera: Nycteridae; left call) is shown next to a call from Megaderma lyra (Yinpterochiroptera: Megadermatidae; right call). Both bats emit brief, broadband multiharmonic signals and also listen for prey-generated sounds to detect and localize prey. Call reconstructions are from illustrations in Jones & Teeling (2006), from Taylor (2000) and from unpublished recordings by G. Jones (R. pearsonii).
The new molecular phylogeny of bats also allows a better understanding of adaptive radiation and convergent evolution in echolocation. Bats in both the Yinpterochiroptera and the Yangochiroptera use a diverse range of echolocation calls, illustrating adaptive radiation, and call plasticity can be remarkably flexible even within species (e.g. Siemers et al. 2001). Regarding convergence, high duty cycle echolocation has appeared twice. This most sophisticated form of biosonar—the use of long constant frequency signals terminated by broadband sweeps—evolved independently in the horseshoe bats (Rhinolophidae: Yinpterochiroptera) and in the moustached bat Pteronotus parnellii in the Mormoopidae (Yangochiroptera; figure 2). These bats compensate for Doppler shifts in a speed-dependent manner during flight. They lower the frequency of the call the faster they fly (Schnitzler 1972), so that echoes return consistently at the frequency of best hearing, the ‘acoustic fovea’ (Schuller & Pollak 1979). Although the echolocation signals and some aspects of auditory physiology show remarkable convergence in horseshoe bats and P. parnellii, substantial differences occur in the organization of the auditory cortex (O'Neill 1995), and in the route of sound transmission—horseshoe bats emit calls through their nostrils, P. parnellii calls are emitted orally (Jones & Teeling 2006).
A similar convergence can be seen in bats in the families Megadermatidae (Yinpterochiroptera) and Nycteridae (Yangochiroptera; figure 2). Bats in these families have large ears, used for listening for prey-generated sounds in cluttered situations (Fenton et al. 1983; Marimuthu & Neuweiler 1987) where echolocation can be ineffective (Arlettaz et al. 2001). The bats emit brief, faint, multiharmonic broadband signals and have elaborate noseleaves (Jones & Teeling 2006). Despite their morphological similarities, the bats in these families are not closely related to one another, and their morphology and their echolocation behaviour show remarkable examples of convergent evolution. In fact, earlier phylogenies based on morphology placed the two families as sister taxa (see Gunnel & Simmons 2005). Convergence in call design is also seen between the neotropical insectivorous bat Myotis nigricans, which forages frequently in edge-and-gap and open habitats, and pipistrelle bats, which exploit similar ecological niches. Both M. nigricans and pipistrelles typically emit narrowband signals when searching for prey in open habitats, switching to more broadband calls with a narrowband tail in edge habitats (Siemers et al. 2001).
Extensive convergence in signal design makes reconstruction of the ancestral state difficult (Jones & Teeling 2006). Tongue clicks used by fruit bats in the genus Rousettus evolved secondarily and do not represent an ancestral state. High duty cycle echolocation with Doppler shift compensation as used by horseshoe bats and P. parnellii is also highly derived. The prevalence of multiharmonic signals in many clades suggests that strong harmonic filtering (e.g. as used by species such as many vesper bats where the fundamental harmonic is dominant) may also be derived. Low duty cycle, multiharmonic calls are likely to have been present through much of the evolutionary history of bats (Jones & Teeling 2006).
8. Future challenges
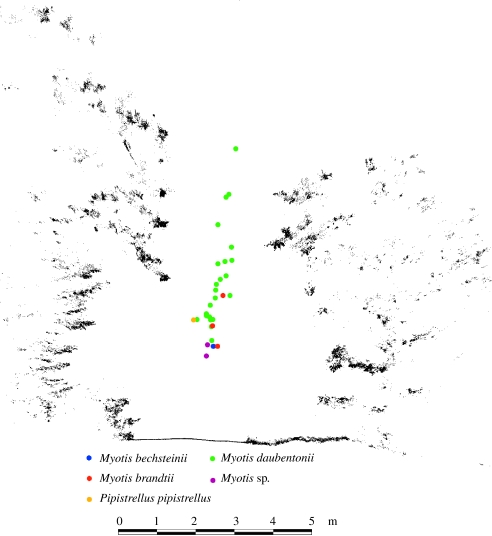
These examples of convergent evolution in echolocation behaviour highlight the importance of environmental factors in shaping bat sonar signals. Bats that feed in similar situations evolve similar designs of echolocation signals despite being distantly related to one another. Physical factors, such as the influence of target size on call frequency, the effect of clutter on bandwidth, the impact of target proximity on pulse duration and the pulse interval all influence the design of bat echolocation signals in ways that can often override phylogenetic constraints. Understanding the genetic factors that underpin the diversity in bat echolocation behaviour has become a tangible challenge now that entire sequences of bat genomes are becoming available (see http://www.genome.gov/11007951). Comparisons of genes that may be associated with audition in bats with those in other mammals may be revealing, and may shed light on some of the mechanisms by which convergence in echolocation strategies is achieved. It is also likely that new understandings in bat echolocation may assist navigation mechanisms in autonomously moving vehicles. The ways in which bats that use broadband signals make subtle adjustments to call design to compensate for Doppler effects may have implications for signal design in vehicles that are able to navigate in darkness (Reynolds 1999). Advances in laser imaging are making three-dimensional reconstructions of the foraging habitats of bats more precise (figure 3), allowing the perceptual challenges faced by bats to be quantified in more realistic perspectives. In combination with three-dimensional reconstruction of the bats' flight routes in the same habitats, this will profoundly improve our understanding of structural and perceptual challenges and behavioural strategies (Aschoff et al. in press).
Figure 3.
Cross-section through laser scan data over a forest track together with positions at which individual bats were flying. The section is perpendicular to the direction of the forest track and the bats' flight direction. Thirty-one flight paths are colour-coded for different species. All fly centrally but most species prefer the more open lower part while Daubenton's bats (Myotis daubentonii) also fly higher in the more confined situation (from Aschoff et al. in press).
References
- Altes R.A, Titlebaum E.L. Bat signals as optimally Doppler tolerant waveforms. J. Acoust. Soc. Am. 1970;48:1014–1020. doi:10.1121/1.1912222 [Google Scholar]
- Arlettaz R, Jones G, Racey P.A. Effect of acoustic clutter on prey detection by bats. Nature. 2001;414:742–745. doi: 10.1038/414742a. doi:10.1038/414742a [DOI] [PubMed] [Google Scholar]
- Aschoff, T., Spieker, H. & Holderied M. W. In press. Untersuchung der Jagdlebensräume von Fledermäusen in Wäldern mit Hilfe von Laserscannern und akustischen Ortungssystemen zur Flugbahnverfolgung. AFZ- DerWald
- Aubauer, R. 1994. Dreidimensionale Flugbahnverfolgung von Fledermäusen Fortschritte der Akustik—DAGA 94 Bad Honnef: DPGGmbH.
- Boonman A.M, Parsons S, Jones G. The influence of flight speed on the ranging performance of bats using frequency modulated echolocation pulses. J. Acoust. Soc. Am. 2003;113:617–628. doi: 10.1121/1.1528175. doi:10.1121/1.1528175 [DOI] [PubMed] [Google Scholar]
- Britton A.R.C, Jones G, Rayner J.M.V, Boonman A.M, Verboom B. Flight performance, echolocation and foraging behaviour in pond bats, Myotis dasycneme (Chiroptera: Vespertilionidae) J. Zool. Lond. 1997;241:503–522. [Google Scholar]
- Dawkins R. Longman Scientific and Technical; Essex, UK: 1986. The blind watchmaker. [Google Scholar]
- Eick G.N, Jacobs D.S, Matthee C.A. A nuclear DNA phylogenetic perspective on the evolution of echolocation and historical biogeography of extant bats (Chiroptera) Mol. Biol. Evol. 2005;22:1869–1886. doi: 10.1093/molbev/msi180. doi:10.1093/molbev/msi180 [DOI] [PubMed] [Google Scholar]
- Fenton M.B, Bell G.P. Recognition of species of insectivorous bats by their echolocation calls. J. Mammal. 1981;62:233–243. doi:10.2307/1380701 [Google Scholar]
- Fenton M.B, Gaudet C.L, Leonard M.L. Feeding behaviour of the bats Nycteris grandis and Nycteris thebaica (Nycteridae) in captivity. J. Zool. Lond. 1983;200:347–354. [Google Scholar]
- Fenton M.B, Portfors C.V, Rautenbach I.L, Waterman J.M. Compromises: sound frequencies used in echolocation by aerial-feeding bats. Can. J. Zool. 1998;76:1174–1182. doi:10.1139/cjz-76-6-1174 [Google Scholar]
- Fullard J.H, Dawson J.W. The echolocation calls of the spotted bat Euderma maculatum are relatively inaudible to moths. J. Exp. Biol. 1997;200:129–137. doi: 10.1242/jeb.200.1.129. [DOI] [PubMed] [Google Scholar]
- Gao W, Hinders M. Mobile robot sonar backscatter algorithm for automatically distinguishing walls, fences and hedges. Int. J. Robot. Res. 2006;25:135–145. doi:10.1177/0278364906059328 [Google Scholar]
- Gunnel G.F, Simmons N.B. Fossil evidence and the origin of bats. J. Mamm. Evol. 2005;12:209–246. doi:10.1007/s10914-005-6945-2 [Google Scholar]
- Heller K.-G, von Helversen O. Resource partitioning of sonar frequency bands in rhinolophoid bats. Oecologia. 1989;80:178–186. doi: 10.1007/BF00380148. [DOI] [PubMed] [Google Scholar]
- Holderied M.W, von Helversen O. Echolocation range and wingbeat period match in aerial-hawking bats. Proc. R. Soc. B. 2003;270:2293–2299. doi: 10.1098/rspb.2003.2487. doi:10.1098/rspb.2003.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderied M.W, Korine C, Fenton M.B, Parsons S, Robson S, Jones G. Echolocation call intensity in the aerial hawking bat Eptesicus bottae (Vespertilionidae) studied using stereo videogrammetry. J. Exp. Biol. 2005;208:1321–1327. doi: 10.1242/jeb.01528. doi:10.1242/jeb.01528 [DOI] [PubMed] [Google Scholar]
- Holderied M.W, Jones G, von Helversen O. Flight and echolocation behaviour of whiskered bats commuting along a hedgerow: range-dependent sonar signal design, Doppler tolerance and evidence for ‘acoustic focussing’. J. Exp. Biol. 2006;209:1816–1826. doi: 10.1242/jeb.02194. doi:10.1242/jeb.02194 [DOI] [PubMed] [Google Scholar]
- Holland R.A, Waters D.A, Rayner J.M.V. Echolocation signal structure in the megachiropteran bat Rousettus aegyptiacus Geoffroy 1810. J. Exp. Biol. 2004;207:4361–4369. doi: 10.1242/jeb.01288. doi:10.1242/jeb.01288 [DOI] [PubMed] [Google Scholar]
- Houston R.D, Boonman A.M, Jones G. Do echolocation signal parameters restrict bats' choice of prey? In: Thomas J.A, Moss C.F, Vater M, editors. Echolocation in bats and dolphins. University of Chicago Press; Chicago, IL: 2004. pp. 339–345. [Google Scholar]
- Hutcheon J.M, Kirsch J.A.W. Camping in a different tree: results of molecular systematics of bats using DNA–DNA hybridization. J. Mamm. Evol. 2004;11:17–47. doi:10.1023/B:JOMM.0000029144.80747.d2 [Google Scholar]
- Jensen M.E, Miller L.A. Echolocation signals of the bat Eptesicus serotinus recorded using a vertical microphone array: effect of flight altitude on searching signals. Behav. Ecol. Sociobiol. 1999;47:60–69. doi:10.1007/s002650050650 [Google Scholar]
- Jones G, Barlow K.E. Cryptic species of echolocating bats. In: Thomas J.A, Moss C.F, Vater M, editors. Echolocation in bats and dolphins. University of Chicago Press; Chicago, IL: 2004. pp. 345–349. [Google Scholar]
- Jones G, Rayner J.M.V. Flight performance, foraging tactics and echolocation in free-living Daubenton's bats Myotis daubentoni (Chiroptera: Vespertilionidae) J. Zool. Lond. 1988;215:113–132. [Google Scholar]
- Jones G, Teeling E.C. The evolution of echolocation in bats. Trends Ecol. Evol. 2006;21:149–156. doi: 10.1016/j.tree.2006.01.001. doi:10.1016/j.tree.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Jung, K., Kalko, E. K. V., & von Helversen, O. In press. Echolocation calls in Central American emballonurid bats: signal design and call frequency alternation. J. Zool. Lond
- Kalko E.K.V, Schnitzler H.-U. Plasticity in echolocation signals of European pipistrelle bats in search flight - implications for habitat use and prey detection. Behav. Ecol. Sociobiol. 1993;33:415–428. doi:10.1007/BF00170257 [Google Scholar]
- Kalko E.K.V, Schnitzler H.U. How echolocating bats approach and acquire food. In: Kunz T.H, Racey P.A, editors. Bat biology and conservation. Smithsonian Institution Press; Washington, DC; London, UK: 1998. pp. 197–204. [Google Scholar]
- Kelly E.J, Wishner R.P. Matched-filter theory for high-velocity, accelerating targets. IEEE Trans. Military Elect. 1965;9:56–69. [Google Scholar]
- Kingston T, Rossiter S.J. Harmonic-hopping in Wallacea's bats. Nature. 2004;429:654–657. doi: 10.1038/nature02487. doi:10.1038/nature02487 [DOI] [PubMed] [Google Scholar]
- Kingston T, Jones G, Zubaid A, Kunz T.H. Resource partitioning in rhinolophoid bats revisited. Oecologia. 2000;124:332–342. doi: 10.1007/PL00008866. doi:10.1007/PL00008866 [DOI] [PubMed] [Google Scholar]
- Kingston T, Jones G, Zubaid A, Kunz T.H. Alternation of echolocation calls in five species of aerial-feeding insectivorous bats from Malaysia. J. Mammal. 2003;84:205–215. doi:10.1644/1545-1542(2003)084<0205:AOECIS>2.0.CO;2 [Google Scholar]
- Lawrence B.D, Simmons J.A. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J. Acoust. Soc. Am. 1982;71:585–590. doi: 10.1121/1.387529. doi:10.1121/1.387529 [DOI] [PubMed] [Google Scholar]
- Marimuthu G, Neuweiler G. The use of acoustical cues for prey detection by the Indian false vampire bat, Megaderma lyra. J. Comp. Physiol. A. 1987;160:509–515. doi:10.1007/BF00615084 [Google Scholar]
- O'Neill W.E. The bat auditory cortex. In: Popper A.N, Fay R.R, editors. Hearing by bats. Springer; New York, NY: 1995. pp. 416–480. [Google Scholar]
- Reynolds, C. W. 1999 Steering behaviors for autonomous characters. In Conf. Proc. 1999 Game Developers Conference, pp. 763–782.
- Schnitzler H.-U. Control of Doppler shift compensation in the greater horseshoe bat, Rhinolophus ferrumequinum. J. Comp. Physiol. 1972;82:79–82. doi:10.1007/BF00714171 [Google Scholar]
- Schnitzler H.-U, Moss C.F, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends Ecol. Evol. 2003;18:386–394. doi:10.1016/S0169-5347(03)00185-X [Google Scholar]
- Schuller G, Pollak G. Disproportionate frequency representation in the inferior colliculus of Doppler compensating greater horseshoe bats. Evidence for an acoustic fovea. J. Comp. Physiol. 1979;132:47–54. doi:10.1007/BF00617731 [Google Scholar]
- Siemers B.M, Schnitzler H.-U. Natterer's bat (Myotis nattereri Kuhl, 1818) hawks for prey close to vegetation using echolocation signals of very broad bandwidth. Behav. Ecol. Sociobiol. 2000;47:400–412. doi:10.1007/s002650050683 [Google Scholar]
- Siemers B.M, Schnitzler H.-U. Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature. 2004;429:657–661. doi: 10.1038/nature02547. doi:10.1038/nature02547 [DOI] [PubMed] [Google Scholar]
- Siemers B.M, Kalko E.K.V, Schnitzler H.-U. Echolocation behavior and signal plasticity in the Neotropical bat Myotis nigricans (Schinz, 1821) (Vespertilionidae): a convergent case with European species of Pipistrellus? Behav. Ecol. Sociobiol. 2001;50:317–328. doi:10.1007/s002650100379 [Google Scholar]
- Speakman J.R, Racey P.A. No cost of echolocation for bats in flight. Nature. 1991;350:421–423. doi: 10.1038/350421a0. doi:10.1038/350421a0 [DOI] [PubMed] [Google Scholar]
- Springer M.S, Teeling E.C, Madsen O, Stanhope M.J, de Jong W.W. Integrated fossil and molecular data reconstruct bat echolocation. Proc. Natl Acad. Sci. USA. 2001;98:6241–6246. doi: 10.1073/pnas.111551998. doi:10.1073/pnas.111551998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Jen P.H.-S. Peripheral control of acoustic signals in the auditory system of echolocating bats. J. Exp. Biol. 1975;69:277–311. doi: 10.1242/jeb.62.2.277. [DOI] [PubMed] [Google Scholar]
- Suga N, Schlegel P. Neural attenuation of responses to emitted sounds in echolocating bats. Science. 1972;177:82–84. doi: 10.1126/science.177.4043.82. doi:10.1126/science.177.4043.82 [DOI] [PubMed] [Google Scholar]
- Taylor P.J. University of Natal Press; Pietermaritzburg, South Africa: 2000. Bats of southern Africa. [Google Scholar]
- Teeling E.C, Scally M, Kao D.J, Romagnoli M.L, Springer M.S, Stanhope M.J. Molecular evidence regarding the origin of echolocation and flight in bats. Nature. 2000;403:188–192. doi: 10.1038/35003188. doi:10.1038/35003188 [DOI] [PubMed] [Google Scholar]
- Teeling E.C, Springer M.S, Madsen O, Bates P, O'Brien S.J, Murphy W.J. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 2005;307:580–584. doi: 10.1126/science.1105113. doi:10.1126/science.1105113 [DOI] [PubMed] [Google Scholar]
- Thabah A, Rossiter S.J, Kingston T, Zhang S, Parsons S, Mya My K, Zubaid A, Jones G. Genetic divergence and echolocation call frequency in cryptic species of Hipposideros larvatus sensu lato (Chiroptera: Hipposideridae) from the Indo-Malayan region. Biol. J. Linn. Soc. 2006;88:119–130. doi:10.1111/j.1095-8312.2006.00602.x [Google Scholar]
- Tian B, Schnitzler H.U. Echolocation signals of the greater horseshoe bat (Rhinolophus ferrumequinum) in transfer flight and during landing. J. Acoust. Soc. Am. 1997;101:2347–2364. doi: 10.1121/1.418272. doi:10.1121/1.418272 [DOI] [PubMed] [Google Scholar]
- Van Den Bussche R.A, Hoofer S.R. Phylogenetic relationships among recent chiropteran families and the importance of choosing appropriate out-group taxa. J. Mammal. 2004;85:321–330. doi:10.1644/1545-1542(2004)085<0321:PRARCF>2.0.CO;2 [Google Scholar]
- von der Emde G, Schnitzler H.-U. Classification of insects by echolocating greater horseshoe bats. J. Comp. Physiol. A. 1990;167:423–430. [Google Scholar]
- Woodward P.M. McGraw-Hill; New York, NY: 1953. Probability and information theory, with applications to radar. [Google Scholar]