Abstract
The extreme thermoacidophiles of the genus Sulfolobus are among the best-studied archaea but have lacked small, reliable plasmid vectors, which have proven extremely useful for manipulating and analyzing genes in other microorganisms. Here we report the successful construction of a series of Sulfolobus–Escherichia coli shuttle vectors based on the small multicopy plasmid pRN1 from Sulfolobus islandicus. Selection in suitable uracil auxotrophs is provided through inclusion of pyrEF genes in the plasmid. The shuttle vectors do not integrate into the genome and do not rearrange. The plasmids allow functional overexpression of genes, as could be demonstrated for the β-glycosidase (lacS) gene of S. solfataricus. In addition, we demonstrate that this β-glycosidase gene could function as selectable marker in S. solfataricus. The shuttle plasmids differ in their interruption sites within pRN1 and allowed us to delineate functionally important regions of pRN1. The orf56/orf904 operon appears to be essential for pRN1 replication, in contrast interruption of the highly conserved orf80/plrA gene is tolerated. The new vector system promises to facilitate genetic studies of Sulfolobus and to have biotechnological uses, such as the overexpression or optimization of thermophilic enzymes that are not readily performed in mesophilic hosts.
INTRODUCTION
Sulfolobus, a genus of thermoacidophilic crenarchaeotes, has provided much of the information currently available on the physiology and molecular biology of archaea from geothermal environments. Seminal studies of Sulfolobus spp. have addressed, for example, chromatin-binding proteins (1,2), replication (3), cell cycle (4), repair (5), transcription (6), translation (7,8) as well as metabolism (9). The genome sequences of three species, Sulfolobus solfataricus, Sulfolobus tokodaii and Sulfolobus acidocaldarius, have been published (10–12). Microarrays for these organisms are commercially available and proteomic studies have been undertaken (13,14).
On a practical level, these advances reflect the relative ease with which Sulfolobus spp. are manipulated in the laboratory. Sulfolobus cells can be grown aerobically and heterotrophically on a variety of complex and defined carbon sources, either in liquid media or on plates, with doubling times as short as a few hours. Since Sulfolobus spp. are hyperthermophiles with optimal growth temperatures around 80°C, their proteins are intrinsically stable and resistant to proteolysis. As a result, Sulfolobus enzymes expressed in mesophilic hosts can often be purified with the aid of a heat step, which removes most proteins of the host. The structural rigidity of thermophilic proteins also appears to be an advantage for crystallization, which is a prerequisite for X-ray analysis of 3D structure.
Ultimately, however, the comprehensive study of molecular phenomena in any organism requires genetic analysis and manipulation in vivo. Although numerous plasmids and viruses have been reported in Sulfolobus spp., the development of these natural genetic elements into experimentally useful tools for Sulfolobus has lagged behind the corresponding progress made with methanogenic and halophilic archaea (15). Although different plasmid and virus-based vectors have been constructed (16–21), to our knowledge only SSV1-based viral vectors (20–22) have been successfully applied by other groups to analyze genes in vivo.
We used the plasmid pRN1 as starting point to construct shuttle vectors for Sulfolobus. This plasmid is notable for its relatively small size (5.4 kb), copy numbers ranging from 10 to 20 in mid-log phase, and the three genes (orf56, orf80 and orf904) that are conserved in other Sulfolobus plasmids. In previous work, we have analyzed the three conserved proteins (representing two DNA-binding proteins and the replication protein) and the transcriptional activity of the plasmid (23–25). Those studies revealed that ORF56 binds upstream of its own gene and down-regulates the expression of the co-transcript orf56/orf904 (26). It therefore appears that ORF56 could be involved in regulating the copy number of the plasmid. Similarly, ORF80 is a sequence-specific DNA-binding protein, but the physiological function of this protein has remained unclear. Orf904 encodes the third conserved protein, a multifunctional 110-kDa replication enzyme that appears to play a central role in replication. However, neither the exact molecular mechanism of replication nor the replication origin is known for pRN1.
These questions would be open to experimental study in vivo by the availability of successful Escherichia coli–Sulfolobus shuttle vectors, but the lack of this information also makes it difficult to predict which features of pRN1 must be preserved in constructing such vectors. We therefore took an empirical approach, in which an artificial transposon was inserted at many locations around pRN1. The resulting plasmids provided a series of potential shuttle vectors differing only in the relative location and orientation of inserted genes, from which the best-performing constructs were identified. The series also provided a way to reveal experimentally which regions of pRN1 may be important for successful propagation in Sulfolobus hosts.
MATERIALS AND METHODS
Strains and growth conditions
This study used S. solfataricus strains PH1-16 (27) and PBL2025 (28), S. islandicus strains REN1H1 (29) R1, R20, S1, R1S1 and HVE10/4 H1 (this study), and S. acidocaldarius MR31 (30). Liquid cultures were grown in Brock's basal salts medium at pH 3.5 (31) or the mineral base of Grogan and Gunsalus (32), supplemented with different carbon and nitrogen sources as indicated. Acid-hydrolyzed casein, i.e. NZAmine AS (Sigma), or enzymatically hydrolyzed casein, i.e. tryptone (BD Biosciences), were added at 0.1%. d-(+)-xylose was added at 0.2%, and d-(+)-lactose in ‘lactose-only’ medium at 0.4%. For growth of untransformed pyrEF mutant strains PH1-16, R20, S1R1, R1, H1 and MR31, 20 µg ml−1 of uracil was added to the medium. Plates were solidified by addition of 0.6% Gelrite (Sigma) and 10 mM CaCl2. Plates and shake flask cultures were incubated at 75°C.
Random insertion into pRN1
The Tn5-derived transposon TnPA21 (33) was amplified by PCR using a primer (5′-CTGTCTCTTATACACATCT) complementary to the mosaic end sequence, the terminal inverted repeat sequence found on both ends of the transposon. Native pRN1 (accession number NC 001771) was isolated from a culture of S. islandicus REN1H1 containing only the pRN1 plasmid (34) using the Nucleo Spin plasmid extraction Kit (Macherey Nagel). Plasmid preparations from ∼100 ml of culture were combined and ethanol-precipitated to obtain 0.1 µg of pRN1 for the transposition reaction. The transposition reaction was carried out in vitro using the EZ-Tn5 transposase (Epicentre) according to the instructions of the manufacturer. Transposon and plasmid were mixed at a molar ratio of 1:1. The transposition reaction products were transformed into E. coli EC100 pir+ (Epicentre). The resulting transformants were screened for correctly inserted transposons by restriction digestion with SacI and NotI. Twenty percent of the screened colonies (a total of 80 plasmids) showed two restriction bands with a combined length of 7.2 kb and were kept for further analysis.
Construction of pA–pN and pJlacS
Thirteen of the 80 constructs were chosen after more precise mapping of the insertion sites by restriction digestion. From these, the E. coli replicon (R6Kγ origin of replication and cat gene) introduced by TnPA21 was excised using the NotI and PspOMI sites present on the ends of the transposon sequence. The resulting pRN1 fragments interrupted at different sites were then cloned into the NotI linearized vector delta2pyrEF. The plasmid delta2pyrEF is a derivative of pBluescript with the lacZ and f1 origin regions deleted. Specifically, pBluescriptSKII(+) was cut with SspI and KpnI, re-ligated, cut with SacI and SapI and re-ligated. The pyrEF genes from S. solfataricus P2 (plasmid pBSKP-pyrEF, generously provided by Christa Schleper) were cloned into the SalI and PstI sites. For constructs pA to pN the transitional region from the delta2pyrEF part to the pRN1 part was sequenced to determine the exact insertion site and the direction of the transposon insertion and the direction of the cloning into the NotI site of delta2pyrEF. In construct pG, by restriction analysis using HindIII an additional HindIII site was found to be present and confirmed by sequencing. As the pRN1 part was not PCR-amplified, but stems from the native plasmid, we conclude that pRN1 had this mutation already when isolated, or that this mutation was introduced in E. coli during propagation of pG. Except for this point mutation, pG corresponds to the expected sequence.
To construct pJlacS we cloned the tf55αlacS expression cassette (20) into the unique SacII restriction site of pJ.
Methylation of plasmids
For transformation into S. acidocaldarius shuttle constructs were methylated at the N4-position of the inner cytosine residues of GGCC recognition sequences to circumvent restriction by the SuaI restriction enzyme (35). Plasmids were methylated in vivo as previously described (36) by transforming the shuttle constructs into E. coli ER1821 (New England Biolabs) bearing the additional plasmid pM.EsaBC4I (New England Biolabs). Complete methylation was confirmed by the absence of any cutting after incubation with 5 U HaeIII for 1–4 h.
Electroporation
Constructs were electroporated either using a Gene Pulser I or Gene Pulser II (BioRad) following the protocol of Schleper et al. (1992) or using a Gene Pulser Xcell (BioRad) with a constant time protocol with input parameters 1500 V, 10.2 ms, 2 mm cuvettes or using the protocol described by Kurosawa and Grogan (36) (1250 V, 1000 Ω, 25 µF, 1 mm cuvettes). For S. acidocaldarius, regeneration was done for 30–40 min in tryptone/xylose medium, water or recovery solution before plating on tryptone/xylose plates or NZAmine/xylose plates. Best results were obtained with recovery solution. Recovery solution was prepared as a 2× concentrated solution (=1% sucrose, 20 mM β-alanine/1.5 mM malate buffer, pH 4.5, 10 mM MgSO4). Directly after electroporation the 50 µl cell suspension was mixed in the cuvette with 50 µl of 2× recovery solution (room temperature), transferred into a 1.5-ml tube and incubated for 30 min at 75°C in a benchtop shaker at 600 r.p.m. before plating the cells. For lactose utilization in S. solfataricus, plating after electroporation was not feasible. Instead, electroporated cells were regenerated for 10 min in 1 ml of Millipore water (pre-warmed) at 75°C and then directly transferred into pre-heated lactose medium and cultivated in 50 ml flasks.
Retransformation
One microliter of genomic DNA preparation or 1–5 µl of plasmid prepared from Sulfolobus by alkaline lysis were transformed into RbCl-competent E. coli XL1-Blue cells or into the mcrBC deficient E. coli strain ER2267 (New England Biolabs).
Plasmid copy number determination
Copy numbers of the different shuttle constructs were determined as already described (26) by qPCR and cell number determination through plating, respectively.
Southern blots
Genomic DNA was prepared from 1 ml of culture using the Chemagic DNA Bacteria Kit (Chemagen, Baesweiler, Germany) according to the instructions of the manufacturer. After digestion with either HindIII for constructs pA–pN or SacI for pJlacS restriction fragments were resolved in 1% agarose gels, transferred to a Hybond N membrane (Amersham) by capillary transfer, fixed by UV irradiation for 5 min on a UV transilluminator and hybridized to digoxigenin-labeled probes complementary to the pyrE gene (position 9–320 from the start of the pyrE gene from S. solfataricus P2) and pRN1 (position 4892–5048 in pRN1) for pA to pN or lacS (position 1124–1438 from the start of the lacS gene from S. islandicus REN1H1) and pRN1 for pJlacS. Labeling, hybridization (50% formamide, 42°C), washing (0.5× SSC, 60°C) and detection was done using the PCR DIG Probe Synthesis Kit and the Digoxigenin Labeling and Detection Kit (Roche).
Colony hybridization
Colonies from plates were transferred to Hybond N membranes and subsequently incubated for 10 min on a filter paper soaked with 0.5 M NaOH, 1.5 M NaCl then for 10 min on a filter paper soaked with 1 M Tris–HCl (pH 7.5), 1.5 M NaCl, then for 5 min on a filter paper soaked with 10× SSC. Membranes were cross-linked for 5 min on the 10× SSC filter paper using a transilluminator. Hybridization and detection were done as described for Southern blots with pRN1-specific probes.
β-Galactosidase assay
For convenience, the broad-specificity β-d-glycosidase (37,38) encoded by the S. solfataricus lacS gene was assayed as β-galactosidase activity. Crude extracts were prepared by a freeze–thaw method (20) in which cells were re-suspended in 50 mM Na-phosphate buffer, pH 7, and subjected to five freeze–thaw cycles (−196°C/+50°C). After centrifugation for 30 min at 13 000 r.p.m. the supernatant was stored at −20°C, or assayed directly. All β-galactosidase assays were conducted in triplicate in a 75°C bench top shaker. The reaction mixture consisted of 1 µl of crude extract (or water for blanks), 92 µl of 50 mM Na-phosphate buffer, pH 7 and the assay was started by addition of 7 µl of 12 mg ml−1 ortho-nitrophenyl-β-d-galactopyranoside (ONPG) solution. Incubation was continued for 5 min before the tubes were rapidly cooled on ice and 100 µl of 1 M Na2CO3 solution was added to stop the reaction. Concentration of ONPG was subsequently determined in a 96-well plate in a plate reader at 410 nm using a standard curve generated with ONPG. Protein concentration of the crude extracts was determined by the method of Ehresmann (39).
X-gal staining
A qualitative β-galactosidase assay was based on hydrolysis of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal). For liquid cultures, 200 µl of culture were mixed with 20 µl of substrate solution (20 mg/ml in dimethylformamide) and incubated at 75°C until color development was observed. To score colonies, plates were sprayed with the same X-gal solution and incubated at 75°C.
Stability measurements
Small cultures (0.2-ml each) were produced under selective conditions by transferring colonies from selective plates to xylose/tryptone medium without uracil. After 2 days of incubation, the cultures (n = 2 to 4 per construct) were sampled, diluted in sterile buffer and plated on plates with and without uracil supplementation; from the resulting colony counts, the numbers of Pyr+ and Pyr− cells in the population was determined. The process was repeated after two cycles of transfer to uracil-supplemented liquid medium (3% inoculum), each involving growth to a final density of ∼4 × 108 CFU/ml. This resulted in a total of three measurements per population and an overall numerical expansion under non-selective conditions of ∼103.
RESULTS
Construction of shuttle vectors
In principle, shuttle vectors can be constructed from two plasmids that replicate in different hosts simply by fusing them at two points that preserve all the important functions of each plasmid. However, in the case of pRN1, it was not clear which ORFs or intergenic regions may be important for successful replication in Sulfolobus hosts. We therefore used transposition to generate pRN1 constructs interrupted at a number of different sites without regard to the location or its sequence context. From the initial transposition mixture, 13 distinct insertion points were chosen for further development, which included addition of the pyrEF genes of S. solfataricus as selectable marker (Figure 1, Table 1). In addition to providing more chances for a successful construct, this unbiased approach allowed us to evaluate possible differences in the performance of the vector constructs in Sulfolobus. This would provide some of the first functional data regarding which of the conserved open reading frames are important for plasmid replication and maintenance.
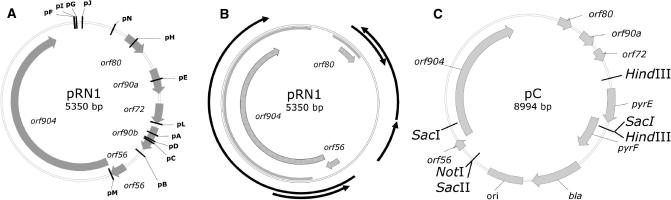
Figure 1.
Physical maps. (A) Positions of the insertion sites of the E. coli replicon and the pyrEF marker genes for shuttle vectors pA–pN. (B): Conserved features of pRN1: thick arrows: conserved open reading frames, gray area: conserved on the nucleotide level within the pRN family plasmids, black arrows: transcripts. (C) Vector map of the shuttle construct pC. Positions of the restriction sites are (clockwise from the top): HindIII (1849), SacI (2792), HindIII (2833), SacII (5374), NotI (5380), SacI (6349).
Table 1.
Insertion sites of the transposon in constructs pA–pN
| Plasmid | Direction of pyrEF genes | Insertion of transposon (position in pRN1) | Interruption | Adjacent unique restriction sites for cloning of inserts |
|---|---|---|---|---|
| pA | + | 1693|1694 | orf90b | NotI/SacII |
| pB | − | 1940|1939 | intergenic | NotI/XmaI |
| pC | + | 1778|1779 | orf90b | NotI/SacII |
| pD | + | 1758|1759 | orf90b | NotI/XmaI |
| pE | + | 1098|1099 | orf90a | NotI/SacII |
| pF | − | 5284|5283 | intergenic | NotI/SacII |
| pG | + | 5309|5310 | intergenic | NotI/SacII |
| pH | + | 621|622 | orf80 | NotI/SacII |
| pI | − | 5290|5289 | intergenic | NotI/XmaI |
| pJ | + | 12|13 | intergenic | NotI/SacII |
| pL | − | 1573|1572 | orf72 | NotI/XmaI |
| pM | + | 2334|2335 | orf56/orf904 | NotI/SacII |
| pN | + | 355|356 | intergenic | NotI/SacII |
Direction of the pyrEF genes (+ same direction as orf56/orf904) as well as two unique adjacent restriction sites are given.
As we expected the replication operon orf56/orf904 to be essential, only one construct interrupted within this region was chosen for analysis. The other constructs were chosen to have the interruption sites distributed as evenly as possible over the remaining part of pRN1. The open reading frames orf80, orf90a, orf72 and orf90b are also interrupted in at least one construct. We have already shown that orf90a, orf72 and orf90b are very unlikely to play a role in plasmid replication or maintenance in view of their very low levels of expression (26).
Recipient strains
The plasmids pA–pN were electroporated into Sulfolobus strains representing different species. As the S. solfataricus pyrEF genes provide the selectable marker, stable uracil auxotrophs were needed as recipient strains. Table 2 gives an overview of the Sulfolobus pyrEF mutants tested as recipients for the various pRN1 constructs.
Table 2.
Sulfolobus species and strains tested as recipient strains for the shuttle constructs pA–pN
| Sulfolobus species and strain | Mutant name | Gene | Type of mutation | Reference | Successful with | |
|---|---|---|---|---|---|---|
| S. acidocaldarius | MR31 | pyrE | Deletion | 18-bp deleted | (30) | pA–pN, pJlacS |
| S. islandicus REN1H1 | R1 | pyrE | Point mutations | S. Berkner and G. Lipps, unpublished results | ||
| S. islandicus REN1H1 | R20 | pyrEF | Insertion sequence | SMN1 in promoter region | (43) | |
| S. islandicus REN1H1 | S1R1 | pyrEF/lacS | Point mutations/ frame shift | S. Berkner and G. Lipps, unpublished results | ||
| S. islandicus HVE10/4 | H1 | pyrEF | Point mutations | S. Berkner and G. Lipps, unpublished results | ||
| S. solfataricus P1 | PH1-16 | pyrF/lacS | Insertion sequences | ISC1359 ISC1217 | (27) | |
| S. solfataricus 98/2 | PBL2025 | lacS | Deletion | SSO3004-SSO3050 deleted | (40) | pJlacS |
For S. solfataricus PH1-16 and the different S. islandicus mutants, the vectors seemed to be unstable, as only very low amounts of shuttle vector could be detected in some experiments. We did observe growth under selective conditions and positive PCR reactions with pRN1-specific primer pairs, but never observed positive results in Southern blots. Thus, the transformed cells seemed to lose the vector rapidly, and the continued growth observed on uracil-free medium may have been due to reversion, or recombinational conversion of the pyrEF mutations to the wild-type sequence.
For S. acidocaldarius MR31, electroporation yielded distinct, rapidly growing colonies on uracil-deficient plates, and growth of these primary transformants was maintained in uracil free-liquid medium (tryptone/xylose or NZAmine/xylose). Sequencing of the chromosomal pyrE locus revealed that no reversion of the mutated pyrE sequence had occurred in these clones, consistent with the nature of this mutation (an 18-bp deletion). Only one shuttle construct, pM (disrupted replication operon orf56/orf904), consistently failed to yield Pyr+ transformants in MR31.
Shuttle constructs are stable and do not integrate or rearrange
Using Southern blots we examined whether the shuttle constructs pA–pN were present in the S. acidocaldarius clones selected after electroporation (Figure 2A). This analysis confirmed that the vector constructs had the correct size and did not integrate into the host genome, as no bands in addition to the expected ones for the episomal form of the vectors were observed. In addition, none of the vector constructs were observed to undergo large rearrangements in S. acidocaldarius, with the exception of pB. For this construct, an additional band of ∼6 kb was observed in the Southern blot, which indicated a rearrangement occurring in the Sulfolobus host. The Southern blot was repeated with a second enzyme (SacI) and again we did not have any indication for rearrangement of the plasmid constructs except pB or for integration into the host genome (data not shown).
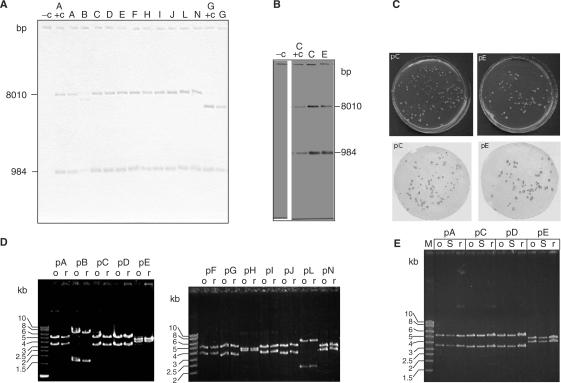
Figure 2.
Analysis of S. acidocaldarius transformants. (A) Southern blot of HindIII digested genomic DNA preparations; the probes complementary to pyrE and a pRN1-fragment (see the Materials and Methods section) were used concurrently. −c: untransformed MR31, A+c: positive control: plasmid pA from E. coli, G+c: separate positive control (plasmid pG from E. coli) because of the additional HindIII restriction site present in pG. (B) Southern blot like in A for controls and cultures transformed with pC and pE after 200 generations of consecutive cultivation. (C) Colony hybridizations for S. acidocaldarius cultures transformed with pC and pE with pRN1 specific probes. (D) Restriction analysis (SacI) of retransformation experiments for all shuttle constructs, o: original plasmid prepared from E. coli, r: retransformed plasmid. (E) Restriction analysis (SacI) of shuttle vectors isolated directly from S. acidocaldarius: o: Plasmid from E. coli, S: plasmid from Sulfolobus (∼40 times more concentrated than from E. coli), r: retransformed plasmid.
Next, we tested by retransformation experiments if the original shuttle plasmids could be recovered intact from S. acidocaldarius transformants. As shown in Figure 2D, 2 to 30 retransformants per construct were checked by restriction analysis and only the correct restriction pattern was observed. In the case of pA, pC, pD and pE, the shuttle plasmids were also isolated directly from transformed Sulfolobus cultures and analyzed by restriction digestion (Figure 2E).
From the initial set of constructs, plasmids pC and pE were chosen to evaluate long-term stability under selective conditions. Cultures of pC and pE transformants were cultivated continuously for ∼200 generations without uracil supplementation. Then retransformation experiments and Southern blots were repeated (Figure 2B), with the same results.
The direction of the insertion in a given region does not influence performance of the vector. The vectors pF, pI and pG, for example, have insertion sites within 15 nt of each other. In pG, the pyrEF genes are oriented clockwise, in pI and pF counter clockwise, without detectable effects on plasmid stability or growth (Figure 3). In addition, the growth phenotype of transformed cells is comparable to that of the untransformed recipient strain when supplemented with uracil.
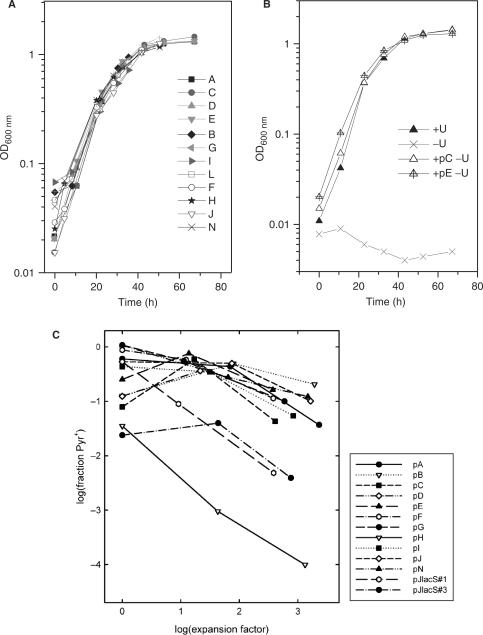
Figure 3.
Growth of transformants. (A) Growth curves for MR31 transformed with pA to pN. (B) Growth curves for the recipient strain MR31 without addition of uracil (U), with the addition of uracil and transformed with pC and pE. (C) Retention of the shuttle vectors under non-selective conditions.
To test if the selection for uracil prototrophy ensures that every cell contains a shuttle vector, cells were plated on selective NZAmine/xylose medium and on non-selective tryptone/xylose medium supplemented with uracil. In eight different experiments comparable colony numbers were obtained on selective and non-selective plates showing that no cells escaped the selection. To prove that the vast majority of cells contained a shuttle vector, cells transformed with constructs pC and pE were plated on non-selective plates and examined by colony hybridizations with pRN1 specific probes (Figure 2C).
Vector retention and copy number
The facile generation of Pyr+ colonies by electroporation and direct plating on selective medium indicated that all the constructs tested, except for construct pM, could replicate in S. acidocaldarius under appropriate selection. In order to provide a more stringent and quantitative comparison of these constructs, we monitored their retention in populations growing in non-selective, uracil-supplemented liquid medium. Specifically, the fraction of Pyr+ cells in the population at three different times was determined, by dilution and plating on uracil-supplemented and unsupplemented plates.
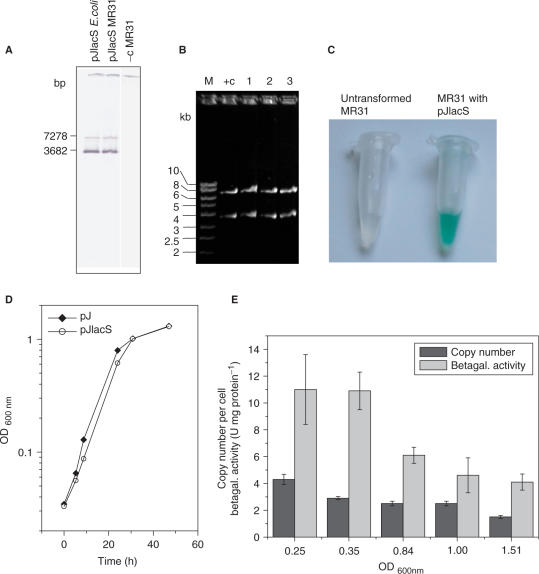
Figure 3C shows the retention of 13 constructs over ∼10 generations, corresponding to ∼1000-fold numerical expansions of the host cell populations. Most constructs showed measurable loss under these conditions, resulting in ∼10% Pyr+ cells in the cultures. In a few cases, however, plasmid retention was much lower. The most severe instability was seen in construct pH, in which the orf80 gene is interrupted. This result provided evidence that the small DNA-binding protein encoded by orf80 has an important role in the stable maintenance of pRN1 and related plasmids. Intermediate instability was observed for construct pJlacS (described below). We suspect that this construct with the very strong tf55α promoter is a burden for the cell. Both pJlacS and pH yielded small or heterogeneous colonies when streaked on selective plates, consistent with the observed instability under non-selective conditions.
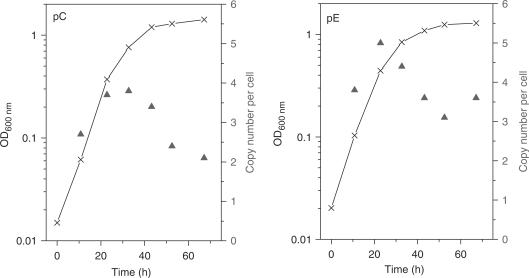
According to qPCR results, all constructs showed copy numbers within the range of 2–8 copies per cell, except for pB that showed low copy numbers around one. For pC and pE, the time course of the copy number during batch fermentation was also determined (Figure 4). The copy number increased in early and mid-log phase and decreased in stationary/death phase. This behavior has also been observed for the wild-type pRN1 plasmid (26). The copy number of the wild-type plasmid in its original host strain (together with pRN2) is higher, reaching 20 copies per cell when grown on rich media containing yeast extract (26) and 10 copies per cell when grown on tryptone media. When pRN1 alone is present in its original host (34) the copy number is only about two. The shuttle vectors therefore maintain a similar copy number in S. acidocaldarius as the native plasmid pRN1 in the original host strain S. islandicus REN1H1.
Figure 4.
Plasmid copy number per cell (triangles) for MR31 transformed with pC (left panel) and pE (right panel) and corresponding growth curves (line).
Suitability for protein expression or reporter gene tests
To test whether the vector tolerates the insertion of sequences containing expressed Sulfolobus genes we cloned the rather strong tf55α promoter (20) together with the lacS gene into shuttle construct pJ generating the vector pJlacS. The stability of this construct was tested by retransformation into E. coli and Southern blotting (Figure 5A and B). The construct turned out to be stably replicated in S. acidocaldarius. Staining with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) revealed that the β-glycosidase was expressed under the control of the heat shock promoter (Figure 5C). This test could be done without prior isolation of a lacS mutant of strain MR31 because the endogenous β-glycosidase activity is very low, i.e. ∼0.01 U/mg protein, in S. acidocaldarius (37). The enzyme activity was also measured quantitatively (as β-galactosidase) at different ODs of a MR31 culture transformed with pJlacS. Copy numbers were determined simultaneously and it was found that measured β-galactosidase activities correlated well with vector copy numbers (Figure 5E), as previously observed (16,20). It should be noted that the β-galactosidase activity of MR31 transformed with pJlacS (2–11 U/mg) is much higher than the wild-type β-galactosidase activity of S. solfataricus (0.2 U/mg) (26) and is comparable to the β-galactosidase activity in a viral overexpression system (1.5–5 U/mg) (20).
Figure 5.
Replication of pJlacS in S. acidocaldarius (A) Southern blot (SacI) of plasmid pJlacS from E. coli, MR31 transformed with pJlacS and untransformed MR31 (−c). The pRN1 specific probe detects the 3.7-kb restriction fragment, the lacS probe detects the 7.3-kb fragment. (B) Retransformation of pJlacS (SacI), +c: pJlacS from E. coli, 1,2,3: retransformants. (C) X-gal test with untransformed MR31 and MR31 transformed with pJlacS (10 min at 75°C). (D) Growth curves for MR31 transformed with pJ (control) and with pJlacS. (E) Reporter gene experiment showing copy numbers of pJlacS per cell and the corresponding β-galactosidase activities.
Replication in S. solfataricus
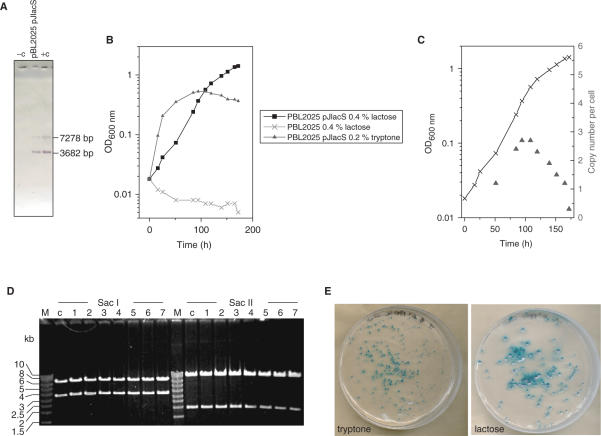
Demonstration of lacS expression in S. acidocaldarius pJlacS transformants suggested the possibility of a selection by conferring, or restoring, the ability to catabolize lactose or other β-glycosides. The S. solfataricus 98/2 lacS deletion mutant PBL2025 (40) was therefore tested for complementation by plasmid pJlacS. After three rounds of selection in liquid medium (0.4% lactose), >95% of all cells contained a shuttle vector, as shown by plating on selective lactose versus non-selective tryptone plates, and X-gal staining of colonies. On both plates equal numbers of colonies were observed, on the non-selective plate in addition to ∼300 colonies also seven white colonies were observed (Figure 6). Southern blot, retransformation, growth and copy number determinations for pJlacS in S. solfataricus are summarized in Figure 6 and indicate that pJlacS is stably replicated in S. solfataricus.
Figure 6.
Replication of pJlacS in S. solfataricus (A) Southern blot (SacI) of untransformed PBL2025 (−c), PBL2025 transformed with pJlacS and pJlacS from E. coli (+c). The pRN1-specific probe detects the 3.7-kb restriction fragment, the lacS probe detects the 7.3-kb fragment. (B) Growth curves of untransformed PBL2025 in 0.4% lactose medium, PBL2025 transformed with pJlacS in 0.4% lactose medium and in 0.2% tryptone medium. (C) Copy numper per cell for pJlacS in PBL2025 in 0.4% lactose medium. (D) Retransformation of pJlacS back into E. coli. (E) Plating of PBL2025 transformed with pJlacS after 100 generations of consecutive cultivation on non-selective tryptone and selective lactose plates stained with X-gal.
Transformation efficiencies
For S. acidocaldarius direct determination of transformation efficiencies is possible, because plating of the primary electroporation mixture can be done after only 30 min of regeneration. Considering all transformations performed in the current study (n = 150), the efficiencies range from 1 × 102 to 6 × 104 transformants per microgram plasmid DNA. The batch of electrocompetent cells, electroporation protocol and regeneration procedure have an influence on the transformation efficiency, as already described (36).
On tryptone/xylose plates, the formation of very small colonies—that did not contain a shuttle vector—was observed in addition to the colonies of normal size that were able to grow in selective liquid medium. Controls without electroporation or without addition of shuttle vector also yielded small colonies, which were not able to grow when re-streaked on selective plates or cultivated in liquid medium. Based on their phenotype and frequency in strain MR31, we hypothesize that these ‘pseudo-transformants’ contain spontaneous mutations elsewhere in the S. acidocaldarius chromosome that partially suppress the pyrE phenotype.
Finally, we confirmed that complete methylation of the shuttle vectors is essential for efficient transformation of S. acidocaldarius. None of the constructs pA–pN yielded transformants when unmethylated or partly protected plasmids were electroporated.
DISCUSSION
Selection
Based on our results with various constructs and recipient strains, we conclude that the primary obstacle to establishing stably replicating shuttle vectors derived from Sulfolobus plasmid pRN1 is not preservation of critical plasmid functions or identification of a required host species, but creation of a suitably reliable selection. For example, point and transposon mutants of S. islandicus or S. solfataricus that showed low reversion frequencies in small scale fluctuation tests (15 × 10−9 – <6 × 10−9 reversions per cell division, unpublished data) displayed for unknown reasons higher reversion frequencies after electroporation with a shuttle construct. These problems could be avoided by the use of a pyrE deletion mutant of S. acidocaldarius. In contrast to S. solfataricus and S. islandicus, background growth on selective plates was not observed with S. acidocaldarius. Although the basis of this difference has not been established, S. acidocaldarius lacks homologs of the cytosine/uracil/thiamine/allantoin permeases (SSO1905, SSO2042) present in S. solfataricus and S. tokodaii (ST1564) that might facilitate growth on medium with very low uracil concentrations.
Essential regions of pRN1
Under selective conditions, the vectors could be faithfully propagated in Sulfolobus. Rearrangements occurred in only two cases, pB and pJlacS, and only after many generations. We do not know why pB behaves differently in this respect, although it is the only construct interrupted in between orf90b and orf56. This region of pRN1 contains several repeats and other remarkable features like a stretch of 17 consecutive C residues (41). An interruption in this region is obviously not as well tolerated as in other regions. The only interruption site that abolished shuttle vector replication completely was that of construct pM, and is situated within the co-transcribed replication operon orf56/orf904. For pM no viable transformants could be isolated. The other conserved open reading frame, orf80, also called plrA (plasmid regulatory), that is present on almost all sequenced genetic elements of Sulfolobus (42) is interrupted in pH. Interestingly, pH shows growth comparable to the other constructs and yields the same transformation efficiencies. Therefore orf80 seems not to be essential for replication of pRN1, at least not when selective pressure is applied. However, under non-selective conditions, construct pH was lost at a much faster rate than any other construct that could be successfully established in S. acidocaldarius. The relative instability of this construct provides the first experimental evidence that the DNA-binding protein ORF80 has an important role in stable maintenance A of pRN1. The instability of this construct may also have practical uses. For example, it may facilitate transfer of pyrEF-marked genes to the host chromosome, by allowing such genes to be first established on an episome, and then stabilized in the population by recombinational integration at the homologous locus.
Stability in E. coli
Many shuttle constructs developed so far for hyperthermophilic archaea have been observed to rearrange in E. coli (18), which hampers the use of these systems. Some of these problems may, in principle, be circumvented by the use of E. coli strains designed specially for dealing with unstable constructs. Additionally reducing the growth temperature to 30°C and using only 50 µg ml−1 of ampicillin is necessary to prevent rearrangements in the pMJ vector system (22). We observed rearrangements for the construct pJlacS in one out of 10 preparations of this plasmid in E. coli XL1-Blue cells at 37°C and 100 µg ml−1 of ampicillin. In general, however, the constructs pA to pN seemed to be fully stable in E. coli. In particular, we never detected rearrangements in retransformed plasmids. In Southern blots, faint traces of plasmid rearrangements were visible for some preparations from E. coli but did not interfere with successful transformation of Sulfolobus.
CONCLUSIONS
We have developed multicopy, non-integrative, plasmid-based Sulfolobus–E. coli shuttle vectors that are very stable in both hosts, and are suitable for the use in protein expression and reporter gene studies. Transformation is rapid and simple, involving electroporation of stable pyrE mutants and plating on uracil-deficient media. The constructs are small, enabling direct cloning into unique SacII/XmaI and NotI restriction sites. The host range so far comprises S. acidocaldarius and S. solfataricus, the two most widely used and best-studied species of Sulfolobus for which genome sequence information is available. The presence of the shuttle constructs in the cells does not cause significant growth retardation and there is limited risk of accidentally contaminating cultures because the vectors are not infectious. It should be emphasized that performance of these shuttle constructs has now been confirmed independently in three different laboratories using slightly different electroporation and cultivation protocols.
In addition, the use of S. acidocaldarius as recipient strain has certain practical advantages which somewhat mitigate the inconvenience of requiring specific DNA methylation. Sulfolobus acidocaldarius does not contain any integrated copies of pRN1 or genes homologous to pRN1 genes. This enables detailed experiments on essential regions and proteins for pRN1 replication and maintenance without interference from plasmid-gene homologs located on the host chromosome. Because of the low sequence similarity between S. acidocaldarius and S. solfataricus there is also minimal risk of undesired homologous recombination when cloning genes of S. solfataricus into the shuttle vector, e.g. for protein expression. Sulfolobus acidocaldarius is the only Sulfolobus species so far that does not contain active insertion sequences and seems to be genetically stable (12). In addition, it is the Sulfolobus species showing the highest growth rate with doubling times of around 3–4 h during exponential growth, and exhibits efficient homologous recombination (36). In this context, the series of pRN1 shuttle vectors we have constructed promises to add detailed genetic analyses to the already advanced biochemical characterization of various Sulfolobus gene products.
ACKNOWLEDGEMENTS
This work was supported by the DFG (Li913/4) in the framework of the Schwerpunkprogramm Archaea to G.L., an NSF grant MCB0543910 to DG and a VIDI grant of the Dutch Science Organization (NWO) to S.-V.A. We are grateful to New England Biolabs for providing us with the plasmid pM.EsaBC4I for methylation of the shuttle constructs, to Christa Schleper for the plasmid pBSKP-pyrEF and the strain S. solfataricus PH1-16, and to Paul Blum for the strain S. solfataricus PBL2025 and Peter Agron for the plasmid pPA21. In addition, DG. thanks R. Cho and C. Sakofsky for communicating unpublished results. Funding to pay the Open Access publication charges for this article was provided by Deutsche Forschungsgemeinschaft (DFG).
Conflict of interest statement. None declared.
REFERENCES
- 1.Agback P, Baumann H, Knapp S, Ladenstein R, Hard T. Architecture of nonspecific protein-DNA interactions in the Sso7d-DNA complex. Nat. Struct. Biol. 1998;5:579–584. doi: 10.1038/836. [DOI] [PubMed] [Google Scholar]
- 2.Bell SD, Botting CH, Wardleworth BN, Jackson SP, White MF. The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science. 2002;296:148–151. doi: 10.1126/science.1070506. [DOI] [PubMed] [Google Scholar]
- 3.Robinson NP, Dionne I, Lundgren M, Marsh VL, Bernander R, Bell SD. Identification of two origins of replication in the single chromosome of the archaeon Sulfolobus solfataricus. Cell. 2004;116:25–38. doi: 10.1016/s0092-8674(03)01034-1. [DOI] [PubMed] [Google Scholar]
- 4.Lundgren M, Bernander R. Genome-wide transcription map of an archaeal cell cycle. Proc. Natl Acad. Sci. USA. 2007;104:2939–2944. doi: 10.1073/pnas.0611333104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling H, Boudsocq F, Woodgate R, Yang W. Crystal structure of a Y-family DNA polymerase in action: a mechanism for error-prone and lesion-bypass replication. Cell. 2001;107:91–102. doi: 10.1016/s0092-8674(01)00515-3. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi SA, Bell SD, Jackson SP. Factor requirements for transcription in the Archaeon Sulfolobus shibatae. EMBO J. 1997;16:2927–2936. doi: 10.1093/emboj/16.10.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condo I, Ciammaruconi A, Benelli D, Ruggero D, Londei P. Cis-acting signals controlling translational initiation in the thermophilic archaeon sulfolobus solfataricus. Mol. Microbiol. 1999;34:377–384. doi: 10.1046/j.1365-2958.1999.01615.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosendal KR, Wild K, Montoya G, Sinning I. Crystal structure of the complete core of archaeal signal recognition particle and implications for interdomain communication. Proc. Natl Acad. Sci. USA. 2003;100:14701–14706. doi: 10.1073/pnas.2436132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouns SJ, Walther J, Snijders AP, van de Werken HJ, Willemen HL, Worm P, de Vos MG, Andersson A, Lundgren M, et al. J. Biol. Chem. 2006;281:27378–27388. doi: 10.1074/jbc.M605549200. [DOI] [PubMed] [Google Scholar]
- 10.She Q, Singh RK, Confalonieri F, Zivanovic Y, Allard G, Awayez MJ, Chan-Weiher CC, Clausen IG, Curtis BA, et al. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl Acad. Sci. USA. 2001;98:7835–7840. doi: 10.1073/pnas.141222098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawarabayasi Y, Hino Y, Horikawa H, Jin-no K, Takahashi M, Sekine M, Baba S, Ankai A, Kosugi H, et al. Complete genome sequence of an aerobic thermoacidophilic crenarchaeon, Sulfolobus tokodaii strain7. DNA Res. 2001;8:123–140. doi: 10.1093/dnares/8.4.123. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Brugger K, Skovgaard M, Redder P, She Q, Torarinsson E, Greve B, Awayez M, Zibat A, et al. The genome of Sulfolobus acidocaldarius, a model organism of the Crenarchaeota. J. Bacteriol. 2005;187:4992–4999. doi: 10.1128/JB.187.14.4992-4999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snijders AP, Walther J, Peter S, Kinnman I, de Vos MG, van de Werken HJ, Brouns SJ, Van der OJ, Wright PC. Reconstruction of central carbon metabolism in Sulfolobus solfataricus using a two-dimensional gel electrophoresis map, stable isotope labelling and DNA microarray analysis. Proteomics. 2006;6:1518–1529. doi: 10.1002/pmic.200402070. [DOI] [PubMed] [Google Scholar]
- 14.Barry RC, Young MJ, Stedman KM, Dratz EA. Proteomic mapping of the hyperthermophilic and acidophilic archaeon Sulfolobus solfataricus P2. Electrophoresis. 2006;27:2970–2983. doi: 10.1002/elps.200500851. [DOI] [PubMed] [Google Scholar]
- 15.Allers T, Mevarech M. Archaeal genetics - the third way. Nat. Rev. Genet. 2005;6:58–73. doi: 10.1038/nrg1504. [DOI] [PubMed] [Google Scholar]
- 16.Aucelli T, Contursi P, Girfoglio M, Rossi M, Cannio R. A spreadable, non-integrative and high copy number shuttle vector for Sulfolobus solfataricus based on the genetic element pSSVx from Sulfolobus islandicus. Nucleic Acids Res. 2006;34:e114. doi: 10.1093/nar/gkl615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannio R, Contursi P, Rossi M, Bartolucci S. An autonomously replicating transforming vector for Sulfolobus solfataricus. J. Bacteriol. 1998;180:3237–3240. doi: 10.1128/jb.180.12.3237-3240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aravalli RN, Garrett RA. Shuttle vectors for hyperthermophilic archaea. Extremophiles. 1997;1:183–191. doi: 10.1007/s007920050032. [DOI] [PubMed] [Google Scholar]
- 19.Contursi P, Pisani FM, Grigoriev A, Cannio R, Bartolucci S, Rossi M. Identification and autonomous replication capability of a chromosomal replication origin from the archaeon Sulfolobus solfataricus. Extremophiles. 2004;8:385–391. doi: 10.1007/s00792-004-0399-y. [DOI] [PubMed] [Google Scholar]
- 20.Jonuscheit M, Martusewitsch E, Stedman KM, Schleper C. A reporter gene system for the hyperthermophilic archaeon Sulfolobus solfataricus based on a selectable and integrative shuttle vector. Mol. Microbiol. 2003;48:1241–1252. doi: 10.1046/j.1365-2958.2003.03509.x. [DOI] [PubMed] [Google Scholar]
- 21.Stedman KM, Schleper C, Rumpf E, Zillig W. Genetic requirements for the function of the archaeal virus SSV1 in Sulfolobus solfataricus: construction and testing of viral shuttle vectors. Genetics. 1999;152:1397–1405. doi: 10.1093/genetics/152.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albers SV, Jonuscheit M, Dinkelaker S, Urich T, Kletzin A, Tampe R, Driessen AJ, Schleper C. Production of Recombinant and Tagged Proteins in the Hyperthermophilic Archaeon Sulfolobus solfataricus. Appl. Environ. Microbiol. 2006;72:102–111. doi: 10.1128/AEM.72.1.102-111.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipps G, Rother S, Hart C, Krauss G. A novel type of replicative enzyme harbouring ATPase, primase and DNA polymerase activity. EMBO J. 2003;22:2516–2525. doi: 10.1093/emboj/cdg246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipps G, Stegert M, Krauss G. Thermostable and site-specific DNA binding of the gene product ORF56 from the Sulfolobus islandicus plasmid pRN1, a putative archael plasmid copy control protein. Nucleic Acids Res. 2001;29:904–913. doi: 10.1093/nar/29.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipps G, Ibanez P, Stroessenreuther T, Hekimian K, Krauss G. The protein ORF80 from the acidophilic and thermophilic archaeon Sulfolobus islandicus binds highly site-specifically to double-stranded DNA and represents a novel type of basic leucine zipper protein. Nucleic Acids Res. 2001;29:4973–4982. doi: 10.1093/nar/29.24.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkner S, Lipps G. Characterization of the transcriptional activity of the cryptic plasmid pRN1 from Sulfolobus islandicus REN1H1 and regulation of its replication operon. J. Bacteriol. 2007;189:1711–1721. doi: 10.1128/JB.01586-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martusewitsch E, Sensen CW, Schleper C. High spontaneous mutation rate in the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by transposable elements. J. Bacteriol. 2000;182:2574–2581. doi: 10.1128/jb.182.9.2574-2581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schelert J, Drozda M, Dixit V, Dillman A, Blum P. Regulation of mercury resistance in the crenarchaeote Sulfolobus solfataricus. J. Bacteriol. 2006;188:7141–7150. doi: 10.1128/JB.00558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zillig W, Kletzin A, Schleper C, Holz I, Janekovic D, Hain J, Lanzendoerfer M, Kristjansson JK. Screening for Sulfolobales, their plasmids and their viruses in Icelandic solfataras. Syst. Appl. Microbiol. 1994;16:609–628. [Google Scholar]
- 30.Reilly MS, Grogan DW. Characterization of intragenic recombination in a hyperthermophilic archaeon via conjugational DNA exchange. J. Bacteriol. 2001;183:2943–2946. doi: 10.1128/JB.183.9.2943-2946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brock TD, Brock KM, Belly RT, Weiss RL. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 32.Grogan DW, Gunsalus RP. Sulfolobus acidocaldarius synthesizes UMP via a standard de novo pathway: results of biochemical-genetic study. J. Bacteriol. 1993;175:1500–1507. doi: 10.1128/jb.175.5.1500-1507.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agron PG, Sobecky P, Andersen GL. Establishment of uncharacterized plasmids in Escherichia coli by in vitro transposition. FEMS Microbiol. Lett. 2002;217:249–254. doi: 10.1111/j.1574-6968.2002.tb11483.x. [DOI] [PubMed] [Google Scholar]
- 34.Purschke WG, Schaefer G. Independent replication of the plasmids pRN1 and pRN2 in the archaeon Sulfolobus islandicus. FEMS Microbiol. Lett. 2001;200:97–102. doi: 10.1111/j.1574-6968.2001.tb10699.x. [DOI] [PubMed] [Google Scholar]
- 35.Grogan DW. Cytosine methylation by the SuaI restriction-modification system: implications for genetic fidelity in a hyperthermophilic archaeon. J. Bacteriol. 2003;185:4657–4661. doi: 10.1128/JB.185.15.4657-4661.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurosawa N, Grogan DW. Homologous recombination of exogenous DNA with the Sulfolobus acidocaldarius genome: properties and uses. FEMS Microbiol. Lett. 2005;253:141–149. doi: 10.1016/j.femsle.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 37.Grogan DW. Evidence that beta-Galactosidase of Sulfolobus solfataricus is only one of several activities of a thermostable beta-d-Glycosidase. Appl. Environ. Microbiol. 1991;57:1644–1649. doi: 10.1128/aem.57.6.1644-1649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbett K, Fordham-Skelton AP, Gatehouse JA, Davis BG. Tailoring the substrate specificity of the beta-glycosidase from the thermophilic archaeon Sulfolobus solfataricus. FEBS Lett. 2001;509:355–360. doi: 10.1016/s0014-5793(01)03154-4. [DOI] [PubMed] [Google Scholar]
- 39.Ehresmann B, Imbault P, Weil JH. Spectrophotometric determination of protein concentration in cell extracts containing tRNA's and rRNA's. Anal. Biochem. 1973;54:454–463. doi: 10.1016/0003-2697(73)90374-6. [DOI] [PubMed] [Google Scholar]
- 40.Schelert J, Dixit V, Hoang V, Simbahan J, Drozda M, Blum P. Occurrence and characterization of mercury resistance in the hyperthermophilic archaeon Sulfolobus solfataricus by use of gene disruption. J. Bacteriol. 2004;186:427–437. doi: 10.1128/JB.186.2.427-437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keeling PJ, Klenk HP, Singh RK, Feeley O, Schleper C, Zillig W, Doolittle WF, Sensen CW. Complete nucleotide sequence of the Sulfolobus islandicus multicopy plasmid pRN1. Plasmid. 1996;35:141–144. doi: 10.1006/plas.1996.0016. [DOI] [PubMed] [Google Scholar]
- 42.Greve B, Jensen S, Brugger K, Zillig W, Garrett RA. Genomic comparison of archaeal conjugative plasmids from Sulfolobus. Archaea. 2004;1:231–239. doi: 10.1155/2004/151926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkner S, Lipps G. An active nonautonomous mobile element in Sulfolobus islandicus REN1H1. J. Bacteriol. 2007;189:2145–2149. doi: 10.1128/JB.01567-06. [DOI] [PMC free article] [PubMed] [Google Scholar]