Abstract
We recently identified circulating osteoblastic cells using antibodies to osteocalcin (OCN) or alkaline phosphatase (AP). We now provide a more detailed characterization of these cells. Specifically, we demonstrate that 46% of OCN positive (OCNpos) cells express AP, and 37% also express the hematopoietic/endothelial marker, CD34. Using two different anti-OCN antibodies and forward/side light scatter characteristics by flow cytometry, we find that OCNpos cells consist of two distinct populations: one population exhibits low forward/side scatter, consistent with a small cell phenotype with low granularity, and a second population has higher forward/side scatter (larger and more granular cell). The smaller, low granularity population also co-expresses CD34, whereas the larger, more granular cells are CD34 negative. Using samples from 26 male subjects aged 28 to 68 years, we demonstrate that the concentration of circulating OCNpos cells increases as a function of age (R = 0.59, P = 0.002). By contrast, CD34pos cells tend to decrease with age (R = −0.31, P = 0.18); as a consequence, the ratio of OCNpos:CD34pos cells also increases significantly with age (R = 0.54, P = 0.022). These findings suggest significant overlap between circulating cells expressing OCN and those expressing the hematopoietic/endothelial marker, CD34. Further studies are needed to define the precise role of circulating OCNpos cells not only in bone remodeling but rather also potentially in the response to vascular injury.
Keywords: Circulating, mesenchymal, osteocalcin, immunophenotyping
Introduction
Pioneering studies by Friedenstein and colleagues [1] established almost 40 years ago that the bone marrow stroma contains plastic adherent cells (colony forming unit-fibroblast) that can give rise to a broad spectrum of fully differentiated connective tissues, including cartilage, bone, adipose tissue, fibrous tissue, and myelosupportive stroma. Since then, there has been an extensive body of work (summarized in [2]) on the characterization of bone marrow stromal cells with osteoblastic potential in rodent and in human systems. Concurrent with this work, however, Long and colleagues identified, over a decade ago, a non-adherent population of cells in bone marrow with osteogenic potential [3, 4]. Rather than selection by adherence to plastic, the primary assay used was cell sorting using antibodies to osteocalcin (OCN), osteonectin, and bone alkaline phosphatase (AP). When cultured in the presence of TGF-β and accessory bone marrow cells (the identity of which still remains unclear), OCN positive (OCNpos) cells proliferated and differentiated into mature osteoblastic cells expressing bone-related genes and capable of mineral deposition [3, 4]. Somewhat surprisingly, despite the fact that OCN is a secreted protein, these investigators did not need to permeabilize the cells in order to detect cells producing OCN. The reasons why this was possible are somewhat unclear, but may have to do with the fact that OCN does possess Gla residues [5], which may allow for at least temporary anchoring of the protein to the cell membrane as the protein is secreted, similar to the mechanisms by which Gla residues on clotting factors allow attachment of these proteins to cell membranes [6]. Alternatively, OCN producing cells may also possess an OCN receptor [7, 8], and as the protein is secreted, it may bind to this cell surface receptor.
The potential functional relevance of these non-adherent osteogenic bone marrow cells remained largely unexplored until recent studies by Dominici and colleagues [9], who compared hematopoietic vs. mesenchymal reconstitution of irradiated mice using either bone marrow stromal or non-adherent fractions. Thus, they obtained plastic-adherent bone marrow stromal cells from FVB/N mice and labeled them with a green fluorescent protein (GFP) marker using a retroviral vector. These cells were then infused into lethally irradiated host mice. As expected, the labeled stromal cells did not contribute to the hematopoietic reconstitution of the host mice, and 0–2% (median, 1.5%) of osteoblasts or osteocytes in the host were GFP positive, indicating limited engraftment of infused bone marrow stromal cells into the host. By contrast, when the identical experiment was repeated using bone marrow non-adherent cells, >90% of blood leukocytes, erythrocytes, and platelets were GFP positive, and even more importantly, up to 50% (median, 18%) of osteoblasts or osteocytes in the host were now GFP positive. Moreover, molecular analysis demonstrated a common retroviral integration site in clonogenic hematopoietic cells and osteoprogenitors from each of seven animals studied, establishing a shared clonal origin for these cell types. These findings thus lent considerable credence to the previous work of Long et al. [3, 4] and established that, at least in the experimental paradigm used by Dominici and colleagues [9], non-adherent bone marrow cells have a >10-fold more robust bone-repopulating activity than do adherent bone marrow stromal cells. Moreover, the findings were also consistent with previous work by Olmsted-Davis et al. [10] suggesting the presence of a unique progenitor cell with both hematopoietic and osteoblastic differentiation potential in the non-adherent subset of bone marrow cells.
The demonstration of potentially functional osteogenic cells in the bone marrow non-adherent fraction raised the obvious question of whether these cells, by virtue of their non-adherent properties, might also be present in the peripheral circulation. Osteoclast precursors had clearly been shown to be present in peripheral blood [11]; while circulating osteoblast precursors, identified on the basis of adherence to plastic, had also been found by several groups [12, 13], the concentration of these cells was extremely low (~1 in 108 mononuclear cells [MNCs] or less in humans) [13]. We reasoned that plastic adherence may well have underestimated the concentration of these cells in peripheral blood, and using flow cytometry following staining with OCN or AP antibodies, recently demonstrated that OCNpos or APpos cells were indeed present in the peripheral blood in humans, constituting ~1% of circulating MNCs [14]. Moreover, the concentration of OCNpos cells was markedly increased (~5-fold) in peripheral blood of adolescent males going through the growth spurt and possibly following fractures. Circulating OCNpos cells also expressed bone related genes (OCN, AP, and collagen I) and formed mineral deposits in vitro and bone in vivo in immunodeficient mice [14], although the relative proportions of the bone formed in vivo that was of donor versus host origin remains to be defined. In the present study, we provide a further characterization of these cells using two different anti-OCN antibodies, address the issue of possible co-expression by OCNpos cells of AP as well as the more primitive endothelial/hematopoietic marker, CD34 [15, 16], and evaluate changes in circulating OCNpos cells with age in adults.
Materials and Methods
Immunohistochemistry of normal human bone
Immunohistochemistry was performed on decalcified normal surgical waste human bone samples using two different anti-OCN antibodies: a polyclonal goat anti-human OCN (SC V-19, Santa Cruz Biotechnology, Santa Cruz, CA) and a monoclonal mouse anti-human OCN (HT AON-5031, Haematologic Technologies, Essex Junction, VT). Sections were deparaffinized and endogenous peroxides were inhibited by 3% H2O2 treatment for 5 minutes. The following procedures were performed on the DAKO autostainer at room temperature: the sections were treated with proteinase K (S3020, Dako Corp., Carpinteria, CA) for 10 minutes for epitope retrieval and than blocked for another 10 minutes in the appropriate serum. Subsequently, slides were incubated for 2 h with either the polyclonal goat anti-human OCN antibody (SC V-19), diluted 1:50 in the blocking solution, or for 30 minutes with the monoclonal mouse anti-human OCN antibody (HT AON-5031), diluted 1:1000. Sections were rinsed with TBST wash buffer and incubated with the appropriate secondary antibody (Zymed Kit for the goat primary antibody, EnVision+ Dual Link System Peroxidase Kit, K4061, DAKO, for the mouse monoclonal antibody). Slides were rinsed in TBST and incubated for 10 minutes with DAB substrate. Schmidts’ hematoxylin was used as a counterstain. To blue the sections, they were rinsed with running tap water, dehydrated through graded alcohol solutions, and cleared in 3 changes of xylene and mounted with a permanent mounting media.
Specimen collection and experimental protocol
For flow cytometry, we analyzed whole blood samples from 11 adult healthy males, aged 28 to 49 years, as well as 16 buffy coat samples from male volunteer donors aged 28 to 68 years obtained from the Mayo Transfusion Center. All studies were approved by the Mayo Clinic Institutional Review Board, and recruited donors of whole blood samples provided written, informed consent. For the buffy coats, only information on age and gender was available on the donors. Whole blood and buffy coat peripheral blood MNC samples were either analyzed with flow cytometry or used for magnetic activated cell sorting (MACS) and/or fluorescent activated cell sorting (FACS). Partial results from the analysis of 11 adult healthy males donors of whole blood samples have been published previously [14].
Isolation of MNCs and immunostaining for flow cytometry
Whole blood or buffy coat samples were layered over Ficoll-Paque density gradients and MNCs isolated and subsequently processed for immunostaining, as previously described [17]. Briefly MNCs recovered from the interface between the blood and Ficoll-Paque were washed three times with ice-cold phosphate buffered saline (pH 7.1) with 0.5% chicken albumin (Sigma), which removed the majority of the platelets. MNCs were counted using a hemocytometer and cell viability measured with trypan blue exclusion. Non-specific binding sites were blocked by incubating MNCs with 10% normal donkey serum (Jackson ImmunoResearch) and 10% human IgG (FcR Blocking Reagent, Miltenyi Biotec) at room temperature for 30 minutes. The MNC density was then adjusted to a final concentration of 107 cells/ml. Aliquots of 100 μl (106 cells) were transferred into 5 ml polystyrene round tubes, incubated for 60 minutes at room temperature with primary antibodies. Primary antibodies consisted of a monoclonal anti-AP (B4–78, BAP Hybridoma Bank, University of Iowa), a polyclonal goat anti-human OCN (SC V-19, Santa Cruz Biotechnology), a monoclonal mouse anti-human OCN (HT AON-5031, Haematologic Technologies, Essex Junction, VT) and a monoclonal mouse anti-CD34 (DAKO A/S DK-2600) antibody. Corresponding control isotype antibodies at the same concentrations as the primary antibodies were used to measure the background staining. After primary antibodies were washed, cell samples were again blocked as described above and incubated with conjugated secondary antibodies at 4°C for 60 minutes. Secondary antibodies included phycoerythrin-conjugated AffinityPure F(ab′)2 fragment donkey anti-mouse IgG (H+L) and FITC-conjugated AffinityPure IgG F(ab′)2 fragment donkey anti-goat (Jackson immunoResearch, West Grove, PA). Cell samples were protected from light, washed once with ice-cold buffer, centrifuged at 4°C at 100 × g and transferred on ice to the flow cytometry Core Facility.
Flow cytometry and data analysis
Cells in suspension were analyzed using a Becton Dickinson FACScan cytometer (Becton Dickinson Immunocytometry Systems) equipped with 488 nm argon laser capable of detecting light scatter (forward and side). Data were analyzed with the WinMDI software and displayed as two-color dot plots of a 530 ± 15 nm bandpass filter (FL1) vs. 585 ± 21 nm bandpass filter (FL2). Electronic compensation was used in the fluorescence channels to remove residual spectral overlap. For each sample, 20,000 events were counted with gates applied on forward/side light scatter over the lymphocyte-monocyte-rich region, as previously described [17]. The frequency of positive cells was measured as the percentile of gated cells in fluorescent channels with activities above 99.5% of the corresponding isotype controls, thus including backgrounds below 0.5%.
Isolation of OCNpos cells using MACS
Following Ficoll extraction, samples of MNCs were blocked and incubated with the either the SC V-19 OCN or the HT AON-5031 anti-OCN antibodies, as described above. Subsequently, the cells were incubated with either the phycoerythrin -conjugated AffinityPure IgG F(ab′)2 fragment donkey anti-goat or the phycoerythrin-conjugated AffinityPure F(ab′)2 fragment donkey anti-mouse IgG (H+L) (Jackson immunoResearch, West Grove, PA) secondary antibody at 4°C for 30 minutes. After an additional washing step, the cells were magnetically labeled with Anti-PE Micro Beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The cell suspension was loaded onto an autoMACS™ cell sorter (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). For the cell separation, the possel_ds program, which is a positive separation program in a sensitive mode and is recommended for isolating cells with low antigen expression and a weak magnetic labeling, was used. The positively selected cells were co-stained with a monoclonal FITC-conjugated mouse anti-CD34 (Clone QBEnd10, DAKO Cytomation, Carpinteria, CA) antibody at 4°C for 30 minutes. The cells were then used in FACS analysis, as described above.
Confocal Microscopy
For confocal microscopy, PE-labelled OCNpos cells isolated by MACS sorting were co-stained with CD34-FITC and were fixed overnight in 4% paraformaldehyde. The following day, the cells were washed with buffer and the cell pellet was resuspended in 50 μl of 1 × PBS with 0.5% BSA and mounted in ProLong® Gold antifade reagent with DAPI (Invitrogen, Eugene, OR) to stain the nucleus. Samples were examined on an LSM510 confocal laser scanning microscope (Carl Zeiss, Inc. Oberkochen, Germany) equipped with an Axiovert 100 M microscope stand and a C-Apochromat 63x/1.2 n.a. water immersion objective lens. The FITC signal was detected with 488 nm excitation from an argon ion laser and images captured through a 505–550 nm bandpass filter. The PE signal was detected with 543 nm excitation from a helium/neon laser and images captured through a 560–615 nm bandpass filter. The DAPI signal was detected with 364 nm excitation from an argon ion laser and images captured through a 385–470 nm bandpass filter.
Statistical Analyses
Unless otherwise indicated, all data are presented as means ± SEM. Statistical comparisons were made using Student’s t-tests. A P value of less than 0.05 was considered significant and all reported P values are two-sided. Linear regressions were used to evaluate correlations between age and percentages of the various cell populations derived from the flow cytometry analysis.
Results
Immunohistochemistry of normal human bone with anti-OCN antibodies
We initially used the anti-OCN antibody utilized in our previous study (SC V-19) [14] and the antibody originally used by Long and colleagues in their work for identifying OCNpos cells in bone marrow (HT AON-5031) [3, 4] and verified that both antibodies stained only mineralized matrix and the appropriate cells in normal human bone (Figure 1A and B). Adjacent muscle or connective tissue of the same section did not stain with the two OCN antibodies (Figure 1C and D) Human bone incubated just with the secondary antibody served as negative control (Figure 1E and F).
Figure 1.
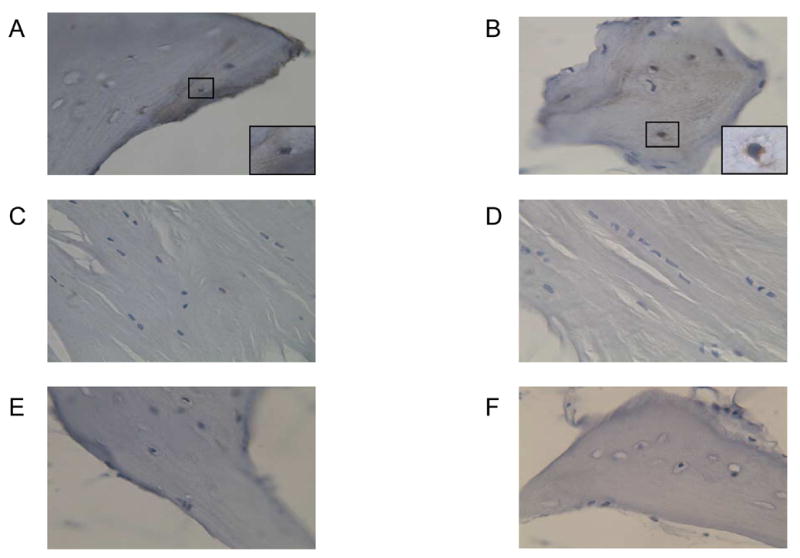
Detection of OCN by immunohistochemistry in normal human bone using the SC V-19 anti-OCN (A, × 40) or the HT AON-5031 anti-OCN (B, × 40) antibodies. Note the brown staining of the matrix and cells with both antibodies. The inserts in A and B show a high power image (× 100) of a positively stained osteocyte in the human bone section. In the same sections, neither antibody stained cells or matrix in the adjacent muscle or connective tissue (C, SC V-19; D, HT AON-5031). Serial sections were also stained with secondary antibody only (E, F). Note the absence of any brown staining in panels C–F; the blue staining is from the Schmidt’s hematoxylin, used to visualize the cells.
Comparison of OCNpos cells isolated with the two anti-OCN antibodies
In order to compare characteristics of cells identified by the SC V-19 versus the HT AON-5031 antibodies, we first isolated OCNpos cells with each antibody using MACS, then co-stained the cells with the anti-CD34 antibody, and subsequently performed flow cytometric analysis on the cells. Using back-gating, we then compared the forward/side scatter characteristics of OCNpos/CD34neg and OCNpos/CD34pos (higher forward scatter indicates larger cells and higher side scatter indicates increased granularity), with the back-gating done only on the population of cells actually expressing OCN and/or CD34 as defined by FACS (i.e., > 99% positive cells). As is evident (Figure 2), regardless of which OCN antibody was used, the OCNpos/CD34neg cells had higher forward and side scatter (larger size and more granularity) than the majority of the OCNpos/CD34pos cells; the latter consisted of two populations: the predominant population had low forward/side scatter, and a minority of cells appeared similar to the OCNpos/CD34neg cells, with higher forward/side scatter Moreover, the forward/side scatter characteristics of the OCNpos/CD34neg and OCNpos/CD34pos cells were very similar with the two antibodies. Additionally, we also used MACS and subsequent FACS (gating on the positive cells, as above) to isolate the two different populations of OCNpos cells (small, low granularity versus larger, more granular) and examined the cells in each population using phase contrast microscopy (Figure 3), confirming the size and granularity characteristics indicated by the flow back-gating. Note that the cells in Figure 3 were isolated regardless of their CD34 status, although based on the findings noted above, the small, round cells in panel A are likely CD34pos, whereas the larger, more granular cells in panel B are likely CD34neg. Finally, since the issue has been raised that circulating OCNpos cells may include osteoclastic cells that express an OCN receptor and bind OCN fragments [18], we performed RT-PCR on sorted OCNpos cells and found that these cells did not express, by RT-PCR, the mRNA for the osteoclast marker, TRAP (data not shown).
Figure 2.
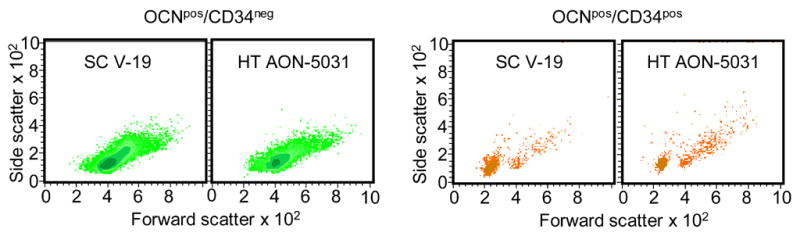
Analysis of cell phenotypes using the SC V-19 anti-OCN and anti-CD34 antibodies. OCNpos cells were first isolated using MACS, then stained with the anti-CD34 antibody, and subsequently analyzed by FACS. OCNpos/CD34neg and OCNpos/CD34pos cells were then back-gated to analyze their forward/side scatter characteristics, with higher forward scatter indicating larger size and higher side scatter indicating more granularity. Shown are density plots, with darker color representing more cells.
Figure 3.

Phase contrast microscopy of cells (× 63) sorted first using MACS and the SC V-19 anti-OCN antibody and then separated by FACS based gating for positive cells and on low forward/side scatter (A) or higher forward/side scatter (B). Consistent with the forward/side scatter characteristics, cells in panel A are smaller and exhibit less granularity than the cells in panel B.
Confocal microscopy
To further characterize the cells stained with the OCN and CD34 antibodies, we performed confocal microscopy following co-staining of cells with the anti-CD34 antibody and either the SC V-19 antibody (Figure 4A–E) or the HT AON-5031 antibody (Figure 4F–J). As is evident, there was surface co-staining of cells with both OCN antibodies and the CD34 antibody. In addition, we also performed confocal microscopy of cells co-stained with the SC V-19 anti-OCN antibody and the anti-AP antibody (Figure 5), demonstrating directly co-staining of the same cell with both antibodies.
Figure 4.
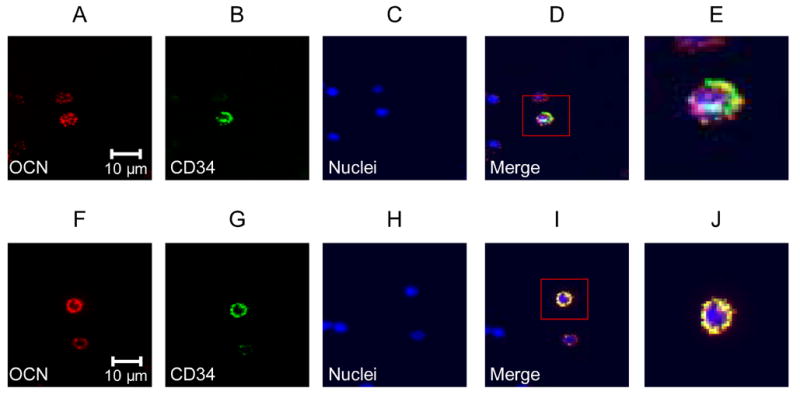
(A–E), confocal microscopy (× 63) using the SC V-19 anti-OCN labeled with a PE-conjugated secondary antibody (red, panel A), the anti-CD34 FITC-conjugated antibody (green, panel B), nuclear stain (DAPI, panel C), the merged image (panel D), and the merged image at higher power (panel E). (F–J), confocal microscopy (x 63) using the HT AON-5031 anti-OCN labeled with a PE-conjugated secondary antibody (red, panel A), the anti-CD34 FITC-conjugated antibody (green, panel B), nuclear stain (DAPI, panel C), the merged image (panel D), and the merged image at higher power (panel E).
Figure 5.
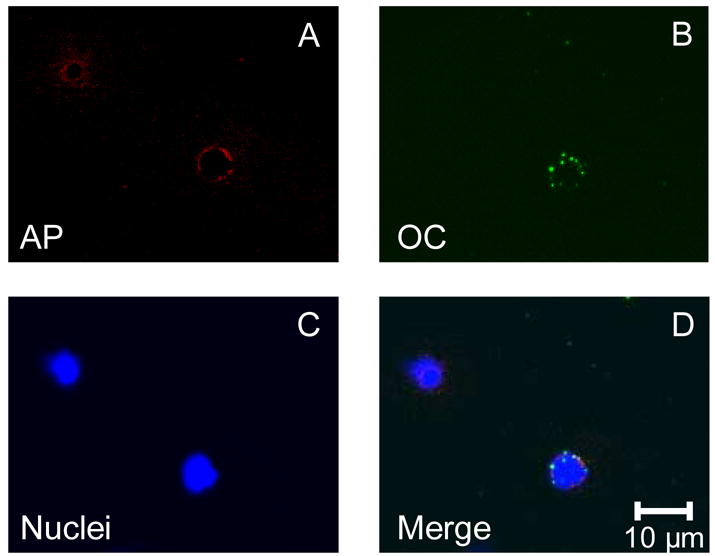
Confocal microscopy (× 63) using the anti-AP antibody labeled with a PE-conjugated secondary antibody (red, panel A), the SC V-19 anti-OCN labeled with a FITC-conjugated secondary antibody (green, panel B), nuclear stain (DAPI, panel C), the merged image (panel D).
Co-staining of OCN with other markers
For the subsequent flow studies, we used the SC V-19 antibody that was utilized in our previous study [14] and assessed co-staining of OCN with AP and CD34. Figure 6 shows representative dot-plots of studies for OCNpos cells expressing AP (Figure 6A) or CD34 (Figure 6B) and AP cells expressing CD34 (Figure 6C). Overall results of the co-staining studies are shown in Table 1. As is evident, 46% percent of OCNpos cells were also APpos, whereas a lower percentage (31%) of APpos cells were OCNpos (P = 0.011). CD34 is generally considered a marker for early hematopoietic/endothelial cells [15, 16] and a somewhat higher percentage (50%) of APpos cells expressed CD34 as compared to OCNpos cells (37%), although this difference was not statistically significant (P = 0.236). Conversely, 30% of CD34pos cells co-stained for OCN, with a somewhat higher percentage (44%) co-staining for AP, but this difference was also not statistically significant (P = 0.115).
Figure 6.
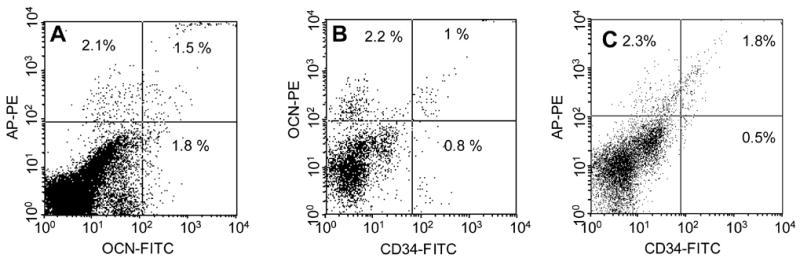
Representative dot plots showing co-staining of (A) OCN with AP; (B) OCN with CD34; and (C) AP with CD34. Percentages refer to the percent of gated cells in each quadrant.
Table 1.
Summary of co-staining data (mean ± SEM). (N) indicates number of independent samples analyzed for each parameter or set of co-staining analyses.
| Percent pos cells in the gated region | Percent of cells in the respective row co-staining for the corresponding marker in the column | |||
|---|---|---|---|---|
| OCN | AP | CD34 | ||
| OCN | 1.2 ± 0.1 (26) | -- | 46 ± 4 (23) | 37 ± 8 (11) |
| AP | 2.5 ± 0.2 (24) | 31 ± 4 (23) | -- | 50 ± 7 (11) |
| CD34 | 1.7 ± 0.4 (21) | 30 ± 6 (11) | 44 ± 6 (11) | -- |
Effects of age on the various cell populations
As shown in Figure 7, in the overall group of subjects, the percentage of OCNpos cells clearly increased with age (R = 0.59, P = 0.002). By contrast, APpos cells did not vary with age (R = 0.08, P = 0.70). There was a weak trend for CD34pos cells to decrease with age (R = −0.31, P = 0.18). As a consequence, the ratio of OCNpos:CD34pos cells also increased significantly with age (R = 0.54, P = 0.022).
Figure 7.
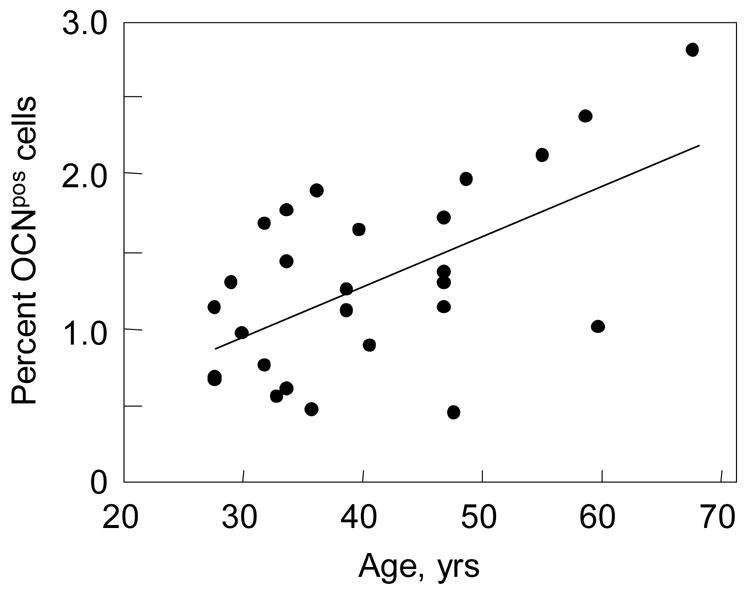
Percent OCNpos cells as a function of age. R = 0.59, P = 0.002.
Discussion
In the present study, we sought to further characterize circulating cells expressing osteogenic markers. We found that OCNpos cells isolated using either the antibody we used in our previous work (SC V-19) [14] or the antibody used by Long and colleagues (HT AON-5031) [3, 4] identified very similar populations of cells, at least as assessed by flow cytometry and confocal microscopy. While additional studies need to be done further characterizing the cells isolated with the two antibodies, these data do provide some reassurance that the cells identified in our previous work using the SC V-19 antibody are not some type of spurious population staining only with this particular antibody.
Coexpression of CD34 by a substantial percentage of OCNpos and APpos cells suggests some degree of overlap between cells staining for osteoblastic markers and hematopoietic/endothelial lineage cells. These findings are consistent with recent work by Pignolo and colleagues [19], who demonstrated the presence of circulating CD34pos cells that expressed bone-related genes and formed bone in an in vivo transplantation assay. These investigators also found that the concentration of these CD34pos osteogenic cells in peripheral blood were increased in patients with recent flares of fibrodysplasia ossifican progressive (FOP) [19]. Of note, recent studies have demonstrated that FOP is caused by an activating mutation in ACVR1, a BMP type I receptor [20]. This mutation may cause activation of an osteogenic program by circulating osteogenic cells and/or result in local tissue cells releasing factors that lead to recruitment and osteogenic differentiation of these circulating cells. However, further studies are needed to address the role of circulating versus local tissue cells in the pathogenesis of FOP.
We also found that, based on forward/side light scatter by flow cytometry, OCNpos cells appeared to consist of two distinct cell populations: a smaller cell population that exhibited low granularity and was CD34 positive and a larger, more granular cell population that was CD34 negative. Of interest, Chen et al. [21] have previously demonstrated that bone marrow CD34pos cells are capable of differentiating into osteoblastic cells in vitro and forming mineralized nodules, although the ability of these cells to form bone in vivo was not tested in that study. These investigators also found that CD34 expression was rapidly lost by these cells in vitro under osteoblast differentiating conditions, suggesting the possibility that the CD34 positive fraction of OCN positive cells may represent a more primitive population, although further work is needed to test this hypothesis.
Our finding that approximately 30% of circulating CD34pos cells were also positive for OCN staining by flow cytometry has recently been independently confirmed by Matsumoto and colleagues [22] who found, using single cell RT-PCR, that 20% of circulating CD34pos cells expressed the OCN mRNA. These investigators also tested the ability of isolated (using MACS) human CD34pos cells to aid in fracture healing in a rat model. Thus, they induced femoral fractures in nude rats and created a fracture non-union model by cauterizing the periosteum around the fracture site. They then infused the rats either with vehicle, unsorted MNCs, or sorted CD34pos cells and demonstrated marked recruitment of CD34pos cells (but not unsorted MNCs) around the fracture site at 7 days following fracture. RT-PCR and immunohistochemical staining at the peri-fracture site demonstrated molecular and histological expression of human-specific markers for endothelial cells (CD31, VE-cadherin) and osteoblasts (OCN, col I) at 14 days following the CD34pos cell infusion. Most remarkably, none of the rats infused with vehicle or with unsorted MNCs healed their fractures, whereas 100% of the rats infused with sorted human CD34pos cells demonstrated healing of the fractures. Based on these findings, these investigators concluded that circulating human CD34pos cells were capable of localizing to a fracture site and contributing both to vasculogenesis and osteogenesis, at least in this particular model.
Our data also demonstrate that the concentration of OCNpos cells in peripheral blood increases with age in men, although the mechanism(s) for this remain unclear. We previously demonstrated a marked increase in circulating concentrations of OCNpos cells in adolescent males going through the growth spurt [14], suggesting that there likely are multiple factors regulating the concentration of these cells in peripheral blood that require further study. Moreover, we focused our present work in males in order to avoid confounding effects of differences in sex steroids between men and women, and studies need to be done to assess the effects of gender on these cell populations and also to evaluate whether there is a similar, age-related increase in circulating OCNpos cells in women.
While the concentration of OCNpos cells increased with age, levels of CD34pos cells tended to decrease, leading to a significant increase in the ratio of OCNpos:CD34pos cells in peripheral blood. Since CD34pos cells include endothelial precursor cells that may be important in the response to vascular injury [16, 23], this increase in the OCNpos:CD34pos ratio in circulating cells raises the possibility that with aging, the response to vascular injury may potentially result in vascular calcification, rather than repair. This possibility, as well as the more general issue of the potential role of circulating osteogenic cells in the response to vascular injury clearly warrants further study.
While a subset of OCNpos cells co-expressed AP (and vice versa), the functions of these two proteins in bone formation may not be required for the same steps in differentiation or at the same sequential step in the process of bone mineralization. Thus, cells staining with either antibody alone or with both antibodies may well be at different stages of osteoblastic maturation, although further studies are needed to characterize these stages.
The origin of these circulating osteoblastic cells remains unclear and also needs to be evaluated. However, recent evidence from Hauge and colleagues that bone remodeling in cortical and in trabecular bone largely occurs in highly vascular bone remodeling compartments (BRCs) that are covered by a layer of bone lining cells [24] (Figure 8) does suggest the possibility that the BRCs may contribute to these circulating cells. In this model, cells destined to become osteoblasts on bone surfaces likely enter the BRC not directly from the bone marrow (which is also simply not possible for BRCs in cortical bone distant from the marrow), but rather via the capillaries that penetrate the BRCs. In addition to the classical bone marrow stromal cells [2], it is possible that bone marrow non-adherent cells, as previously identified by Long et al. [3, 4] access the BRC via this mechanism, or that circulating osteoblastic cells contribute to the pool of osteoblastic cells entering the BRC. On the other hand, given the potential overlap between osteoblastic and endothelial cells noted in the present and previous work [21, 22, 25], and the evidence that vascular pericytes can differentiate into osteoblasts [26], as well as recent work demonstrating the pericytes, themselves, may arise from a CD34pos progenitor in the vessel wall [27], it is also possible that precursor cells in the vasculature (i.e., within the capillary wall penetrating the BRC) give rise to osteoblastic progenitors. Clearly, additional studies are needed to test these hypotheses and to further identify the source of the circulating OCN and AP expressing cells as well as their possible role in bone remodeling. Moreover, the question of whether these circulating cells are the principal cells forming bone either in normal bone remodeling, fracture healing, or vascular calcification, or are recruiting local cells (for example, via the production of BMPs) to form bone (or both) remain unresolved issues and the focus of ongoing work in our laboratory.
Figure 8.

Model of the bone remodeling compartment (BRC) as described by Hauge and colleagues [24]. In this model, osteoblastic cells enter the BRC principally via the afferent capillary and could include non-adherent (or adherent) bone marrow (BM) cells, circulating cells, or osteoblastic cells originating from precursor cells in the vasculature. MSC, marrow stromal cell.
In conclusion, the present study provides a further characterization of circulating cells expressing osteogenic markers. We find evidence for overlap between these cells and cells expressing the hematopoeitic/endothelial marker, CD34. OCNpos cells also appear to increase with age, at least in men. The issue of the origin of these cells and their precise role in bone formation or possibly in vascular calcification remain potentially fruitful areas of further investigation.
Acknowledgments
We would like to thank Dr. B. L. Riggs for helpful comments and discussion.
Footnotes
This work was supported by grants AG004875 and AG024901 (National Institute on Aging) and RR00585 from the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 2.Bianco P, Robey PG. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long MW, Williams JL, Mann KG. Expression of human bone-related proteins in the hematopoietic microenvironment. J Clin Invest. 1990;86:1387–1395. doi: 10.1172/JCI114852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long MW, Robinson JA, Ashcraft EA, Mann KG. Regulation of human bone marrow-derived osteoprogenitor cells by osteogenic growth factors. J Clin Invest. 1995;95:881–887. doi: 10.1172/JCI117738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price PA, Williamson MK, Lothringer JW. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981;256:12760–12766. [PubMed] [Google Scholar]
- 6.Huang M-H, Rigby A, Morelli X, Grant MA, Huang G, Furie B, Seaton B, Furie BC. Structural basis of membrane binding by Gla domains of vitamin D-dependent proteins. Nat Struct Biol. 2003;10:751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 7.Bodine PVN, Komm BS. Evidence that conditionally immortalized human osteoblasts express an osteocalcin receptor. Bone. 1999;25:535–543. doi: 10.1016/s8756-3282(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 8.Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD. Identification of a novel extracellular cation sensing G-protein coupled receptor. J Biol Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici M, Pritchard C, Garlits JE, Hofmann TJ, Persons DA, Horwitz EM. Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. Proc Natl Acad Sci USA. 2004;101:11761–11766. doi: 10.1073/pnas.0404626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olmsted-Davis EA, Gugala Z, Camargo F, Gannon FH, Jackson K, Kienstra KA, Shine HD, Lindsey RW, Hirschi KK, Goodell MA, Brenner MK, Davis AR. Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc Natl Acad Sci USA. 2003;100:15877–15882. doi: 10.1073/pnas.2632959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujikawa Y, Quinn JMW, Sabokbar A, McGee JO, Athanasou NA. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology. 1996;137:4058–4060. doi: 10.1210/endo.137.9.8756585. [DOI] [PubMed] [Google Scholar]
- 12.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Gehron Robey P. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1139. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel DA, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 15.Bender JG, Unversagt KL, Walker DE, Lee W, Van Epps DE, Smith DH, Stewart CC, Bik To L. Identification and comparision of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry. Blood. 1991;77:2591–2596. [PubMed] [Google Scholar]
- 16.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman GC, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 17.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen FH, Lee CJX, Aubin JE. Osteoblasts may take a road well-traveled. BoneKEy-Osteovision. 2005;2:14–18. http://www.bonekey-ibmsorg/cgi/content/full/ibmske;2/9/14.
- 19.Pignolo RJ, Billings PC, Suda RK, Shore EM, Kaplan FS. Circulating osteogenic cells in heterotopic bone formation. J Bone Miner Res. 2004;19(suppl 1):535. (Abstract) [Google Scholar]
- 20.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Consortium TFIR, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 21.Chen JL, Hunt P, McElvain M, Black T, Kaufman S, Choi ES-H. Osteoblast precursor cells are found in CD34+ cells from human bone marrow. Stem Cells. 1997;15:368–377. doi: 10.1002/stem.150368. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, Miwa M, Horii M, Hayashi S, Oyamada A, Nishimura H, Murasawa S, Doita M, Kurosaka M, Asahara T. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–1457. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner N, Kosiol S, Schiegl T, Ahalers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 24.Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16:1575–1582. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- 25.Brandi ML, Collin-Osdoby P. Perspective: Vascular biology and the skeleton. J Bone Miner Res. 2006;21:183–192. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- 26.Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 27.Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol (Cell Physiol) 2005;289:C1396–C1407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]


