Abstract
We have identified a 45-kDa protein purified from rat renal brush border membrane that binds short single-stranded nucleic acid sequences. This activity was purified, reconstituted in proteoliposomes, and then fused with model planar lipid bilayers. In voltage-clamp experiments, the reconstituted 45-kDa protein functioned as a gated channel that allows the passage of nucleic acids. Channel activity was observed immediately after addition of oligonucleotide. Channel activity was not observed in the absence of purified protein or of oligonucleotide or when protein was heat-inactivated prior to forming proteoliposomes. In the presence of symmetrical buffered solution and oligonucleotide, current passed linearly over the range of holding potentials tested. Conductance was 10.4 ± 0.4 picosiemens (pS) and reversal potential was 0.2 ± 1.7 mV. There was no difference in channel conductance or reversal potential between phosphodiester and phosphorothioate oligonucleotides. Ion-substitution experiments documented a shift in reversal potential only when a concentration gradient for oligonucleotide was established, indicating that movement of oligonucleotide alone was responsible for current. Movement of oligonucleotide across the bilayer was confirmed by using 32P-labeled oligonucleotides. Channel open probability decreased significantly in the presence of heparan sulfate. These studies provide evidence for a cell surface channel that conducts nucleic acids.
For many years it has been clear that cellular mechanisms exist that allow a cell to recognize and internalize nucleic acids. For example, the presence of anti-DNA antibodies to host DNA in autoimmunity or after DNA injection into experimental animals (1) and the biological responses of T and B cells to DNA (2) suggest recognition of DNA at the cell surface, most likely through specific receptors. The emergence of systemically administered chemically modified antisense oligonucleotides as a potential therapeutic modality has reinvigorated efforts to define the mechanisms of cellular uptake of DNA. Studies using systemically administered oligonucleotides have provided significant insight into the potential cellular sites for nucleic acid uptake. Oligonucleotides injected into experimental animals are rapidly cleared from plasma and localize primarily to kidney, liver, and spleen (3–7). Although oligonucleotides do not readily cross the blood–brain barrier, they accumulate in neuronal tissue when injected directly into cerebral spinal fluid. Previous studies have established that short single-stranded DNAs are rapidly internalized by a variety of cultured cells (8–16). Several putative DNA binding/transport proteins have been isolated from cell membranes (7, 9, 10, 13, 17–19), and recently an integrin, Mac-1 (19), has been found to potentially mediate endocytosis of nucleic acid on the surface of macrophages. The mechanism of nucleotide internalization in most tissues, however, remains unclear.
The kidney is an important tissue that accumulates oligonucleotides. We previously reported that systemically administered phosphorothioate antisense oligonucleotides are filtered at the glomerulus and undergo receptor-mediated internalization along the length of the tubule (7). This observation led us to affinity purify an oligonucleotide binding protein from renal brush border membranes. In those studies, DNA binding activity was confirmed with electrophoretic mobility shift assays (7). The current studies were initiated to determine whether this DNA binding protein possessed channel and/or transport activity. To accomplish this objective, purified protein was reconstituted in model planar lipid membranes and movement of oligonucleotides across the bilayers was examined.
METHODS
Oligonucleotide Synthesis.
Oligonucleotides were purchased from Oswel DNA Services (Southampton, U.K.). They were synthesized on an Applied Biosystems model 394 DNA synthesizer using Applied Biosystems reagents. The oligonucleotides were purified in a two-step reverse-phase HPLC protocol. After the second HPLC step, collected fractions were pooled, evaporated to dryness, redissolved in 2 ml of distilled water, and freeze-dried to remove excess salt. Residual salt was removed by purifying the oligonucleotide on a NAP-25 column (Sephadex G-25, Pharmacia) according to the manufacturer’s instructions. Purity of oligonucleotide was assessed by capillary zone electrophoresis. If oligonucleotide pools contained smaller synthesis products, the second HPLC purification step was repeated until only full-length oligomer was present. Finally, before use in model lipid bilayer experiments, oligonucleotides were once again desalted with a G-25 spin column (Select-D, G-25, 5 Prime – 3 Prime, Inc.) that had been preequilibrated with the appropriate experimental buffer (experimental buffers are detailed below).
Protein Purification.
Nucleic acid binding/channel protein (NAC) was purified from renal brush border membranes as described (7). In brief, male Sprague–Dawley rats were anesthetized with Inactin and both kidneys were removed. Outer cortical tissue was harvested and cell membranes were separated from other cell components by differential centrifugation. Brush border membranes were separated from basolateral membranes by using a Percoll gradient. Purity of the brush border membrane vesicles was assessed by measuring enrichment of alkaline phosphatase activity (a brush border-specific enzyme) and reduction of Na+K+-ATPase activity (a basolateral specific marker). DNA binding protein was affinity-purified by incubating brush border membrane vesicles with immobilized phosphorothioate oligonucleotide [d(TCCCAGGCTCAGATCTGGTC); S-Oligo]. S-Oligo was immobilized by synthesizing the oligonucleotide with a 5′ biotin and then binding the biotinylated oligonucleotide to streptavidin-coated agarose beads (Pierce). Phosphorothioate-modified oligonucleotides were used in affinity purification to reduce degradation of the DNA. The affinity column was washed several times and bound protein was eluted with 1 M KCl.
Lipid Bilayer System.
Bilayer experiments were performed as described (20, 21). Briefly, affinity-purified NAC was used to form proteoliposomes (PLs) by sonicating purified protein with a 1:1 mixture of bovine brain phosphatidylethanolamine (10 mg/ml) and phosphatidylserine (10 mg/ml). The experimental apparatus consisted of two 1-ml buffer chambers separated by a Teflon film that contained a single 50- to 100-μm hole. A lipid bilayer was formed by “painting” the hole with a 1:1 mixture of phosphatidylethanolamine and phosphatidylserine dissolved in n-decane to a final concentration of 50 μg/ml. This resulted in formation of a high-resistance seal between the two cups. For these studies, the cis side was defined as the chamber connected to the voltage-holding electrode and all voltages are referenced to the trans (ground) chamber.
Stability of the bilayer was determined by clamping voltage at various levels. If a resistance >100 GΩ and noise <0.2 pA were maintained in the patch, NAC-containing PLs (NAC-PLs) were added to the trans chamber. Stability of the bilayer was reexamined and if the membrane remained stable, experiments were initiated by adding oligonucleotide (5 μM). Two oligonucleotides were used in these experiments. They had identical base sequence, but one oligonucleotide consisted of the native phosphodiester backbone (Oligo) and the other oligonucleotide was modified (after synthesis) to replace a nonbridging oxygen of the phosphodiester bond with sulfur (phosphorothioate, S-Oligo). When channel activity was observed, PLs were washed from the trans chamber to limit further channel incorporation. Channel events with an open time greater than 2.0 ms and a noise level at the open state less than two times background noise were analyzed by using commercially available software (pclamp, Version 6.02, Axon Instruments, Foster City, CA). All experiments were performed with either symmetrical buffered solutions or with ionic gradients to examine channel selectivity. Exact composition of solutions used in each experiment is described below.
Channel Blockade.
Studies were performed to determine whether heparan sulfate, a large polyanion similar in molecular weight and valence to Oligo and S-Oligo, altered channel activity. Previous studies have shown that oligonucleotides bind to heparin binding proteins and that heparin sulfate inhibits oligonucleotide entry in cultured cells (19). For these experiments, purified protein was reconstituted in lipid bilayers, oligonucleotide (5 μM) was added to both bilayer chambers, and oligonucleotide-dependent channel activity was documented. Heparan sulfate (average Mr ≈ 7,500; Sigma) was then added in various amounts and channel activity was monitored. Channel kinetics were compared before and after addition of heparan sulfate.
Radiolabeled Oligonucleotide Studies.
Oligo or S-Oligo was 5′ end-labeled with 32P by using polynucleotide kinase. PLs were formed with purified protein and channel activity was established by adding PLs to the trans chamber, adding unlabeled oligonucleotide (5 μM) to both chambers, and clamping holding potential at +100 mV. Once stable channel activity was established, PLs were washed from the trans chamber to limit further channel incorporation and [32P]Oligo was added to the trans chamber (input chamber) to a concentration of 20 nM (3 × 107 cpm). After a minimum period of 20 min, solution from the cis chamber (collection chamber) was collected, radioactivity was measured by liquid scintillation, and the amount of oligonucleotide moving across the bilayer was calculated from the specific activity of the labeled oligonucleotide. If the bilayer broke prior to sample collection, the experiment was terminated without collecting a sample. In three of the experiments, radioactivity was also measured in a fraction of collection chamber solution by scintillation counting, and the remaining sample was used for analysis by gel electrophoresis on 15% polyacrylamide/7 M urea gels to document that the label remained with the full-length oligonucleotide.
RESULTS
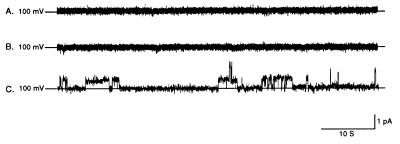
To document formation of channels with affinity-purified protein, experiments were performed by using NAC-PLs in the presence or absence of oligonucleotide. These experiments are summarized in Fig. 1, which depicts representative 1-min current traces. The lipid bilayer was electrically stable and channel activity was not observed when NAC-PLs and oligonucleotides were not present (Fig. 1A). Addition of either NAC-PLs alone (Fig. 1B) or Oligo in the absence of NAC-PLs (data not shown) did not alter stability of the bilayer, and channel activity was not seen under either condition. When Oligo (5 μM) was added in the presence of NAC-PLs, however, gated channel activity was observed (Fig. 1C). The channel gated with clear transitions between 0 current (i.e., channel residing in the closed state, indicated by the solid horizontal line) and approximately 1 pA of current (i.e., the channel residing in the open state) when voltage was clamped at +100 mV. When oligonucleotide was removed from both chambers, channel activity was abolished and reintroduction of Oligo to both chambers restored channel activity (data not shown). Increasing either the amount of NAC used to form the PLs or the amount of NAC-PLs added to the bilayer system resulted in an increase in the number of channels in the membrane and an increase in total current. When NAC was heat-denatured before forming PLs, channel activity was not observed. In additional control experiments, NAC was replaced with an organic acid channel [the expressed recombinant urate transporter/channel protein (21)] or with serum albumin, which is thought to bind nucleic acids but presumably without channel activity. Neither one of these proteins demonstrated oligonucleotide-dependent channel activity (data not shown).
Figure 1.
Representative 1-min current traces from a lipid bilayer experiment in the presence or absence of purified protein and/or oligonucleotide. Purified protein functioned as a gated channel when reconstituted in lipid bilayers and channel activity was observed only when both protein and oligonucleotide were present in the bilayer system. (A) Current trace, obtained at a holding potential of +100 mV, after formation of a lipid bilayer in the absence of both purified protein and oligonucleotide. The lipid bilayer alone was electrically stable and without channel activity. (B) Current trace, obtained at a holding potential of +100 mV, after the addition of NAC-PLs but not oligonucleotide. After the addition of NAC-PLs alone the bilayer remained stable without evidence of channel activity. The bilayer also remained stable after the addition of oligonucleotide in the absence of NAC-PLs (data not shown). (C) Current trace, obtained at a holding potential of +100 mV, after addition of both NAC-PLs and 5 μM Oligo. In the presence of both purified protein and oligonucleotide, channel activity was observed with clear transitions between open and closed states.
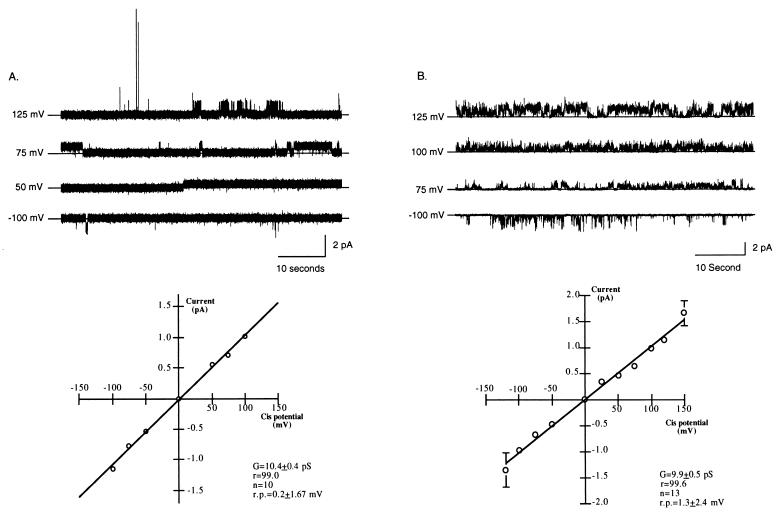
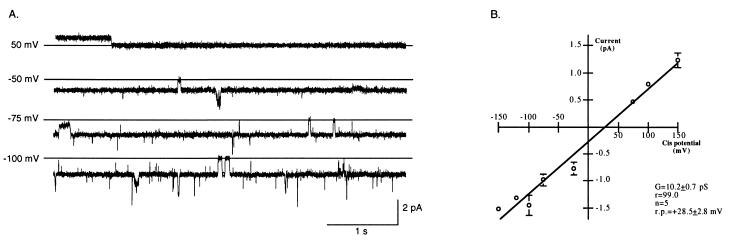
Channel activity was initially characterized with buffered solution (200 mM CsCl/20 mM Tris⋅HCl, pH 7.4) and 5 μM Oligo on both sides of the lipid bilayer (symmetrical solutions). Under these conditions, clear transitions between the open and closed states were evident (Fig. 2A Upper). The channel exhibited no evidence of rectification (Fig. 2A), and there was no apparent dependence of open probability (Po) on voltage (data not shown). Fig. 2A Lower summarizes the current–voltage relationship of the channel in buffered solutions symmetrical for CsCl and Oligo concentrations (n = 10). Under these conditions, the unitary slope conductance was 10.4 ± 0.4 pS and the reversal potential was 0.2 ± 1.7 mV. Histograms of closed and open times suggested that the channel displayed one open and one closed state (data not shown).
Figure 2.
Summary of channel activity in buffered solutions identical in the concentration of salt and either Oligo or S-Oligo on both sides of the membrane (symmetrical buffered solution). Experiments were performed at different holding potentials after addition of identical buffered solution (200 mM CsCl/20 mM Tris⋅HCl, pH 7.4) and either Oligo or S-Oligo (5 μM) to both bilayer chambers. (A) Characterization of the channel in the presence of phosphodiester oligonucleotides (Oligo). (Upper) Representative 1-min traces from a typical experiment with both NAC-PLs and Oligo. For each trace, the holding potential is indicated and the solid horizontal line indicates zero current. (Lower) Current–voltage relationship of the channel in symmetrical Oligo and electrolyte solution. The data are the mean ± SEM of 10 experiments; error bars that are not visible are included within the symbol. The line represents a best fit linear regression analysis. The change in current was linear over the range of holding potentials tested with no sign of rectification. Reversal potential in symmetrical solutions was 0.2 ± 1.67 mV and conductance was 10.4 ± 0.4 pS. G, conductance; r.p., reversal potential; n, number. (B) Characterization of the channel in the presence of phosphorothioate oligonucleotides (S-Oligo). (Upper) Representative 1-min traces from a typical experiment with S-Oligo. For each trace, the holding potential is indicated and the solid horizontal line indicates zero current. (Lower) Current–voltage relationship of the channel in symmetrical S-Oligo and electrolyte solution. The data are the mean ± SEM of 13 experiments; error bars not visible are included within the symbol. Reversal potential in symmetrical solutions was 1.3 ± 2.4 mV and conductance was 9.9 ± 0.5 pS. There was no difference in channel conductance or reversal potential between S-Oligo- and Oligo-stimulated channel activity.
These initial experiments were performed by using oligonucleotides with a phosphodiester backbone. Subsequently, we evaluated NAC characteristics by using phosphorothioate oligonucleotides in symmetrical buffered solution. In 13 experiments using S-Oligo, reversal potential and conductance were not different from that observed when using phosphodiester oligonucleotides (Fig. 2 B vs. A). Under these conditions, unitary slope conductance was 9.9 ± 0.5 pS and reversal potential was 1.3 ± 2.4 mV. Thus, these data suggest that NAC functions as a channel when reconstituted in a lipid bilayer and that both phosphodiester and phosphorothioate oligonucleotides serve as substrates for the channel. To reduce the chance of nucleic acid degradation during subsequent experiments and because there was no difference in channel activity in the presence of either Oligo or S-Oligo, the remainder of the experiments were performed with S-Oligo.
To determine channel selectivity, reversal potential was measured when a 1,000-fold gradient for oligonucleotide (1,000:1 cis chamber to trans chamber) was established across the bilayer. All other components of the buffered solution were symmetrical across the bilayer. Creation of a gradient for S-Oligo resulted in a significant shift in reversal potential from 1.3 ± 2.4 mV (in symmetrical solutions of CsCl and S-Oligo) to 28.5 ± 2.8 mV (P < 0.0001, Figs. 2 vs. 3). Channel activity was observed when holding potential was 0 mV. Furthermore, reversal of the S-Oligo gradient (trans to cis) reversed the current at 0 mV holding potential. By using the Nernst equation and assuming that the oligonucleotide carries a net charge of −20, the predicted reversal potential in the presence of a 1,000-fold gradient for S-Oligo would be +9 mV. The observed reversal potential, however, was approximately 3 times the predicted value. Because reversal potential increases as valence decreases, it is likely that some of the charge of the oligonucleotide was buffered or masked. The reversal potential and current observed at 0 mV holding potential were dependent upon the orientation of the oligonucleotide gradient alone, suggesting that S-Oligo moved through the channel.
Additional experiments were performed to determine whether potassium, cesium, or chloride were also conducted by the channel. Neither current nor channel kinetics were affected by 10-fold gradients for any of these ions (data not shown). This finding suggests that cesium, potassium, and chloride do not contribute significantly to the current observed in the presence of S-Oligo. Finally, reversal potential was unchanged in the presence of a 10-fold gradient for potassium, cesium, or chloride and symmetrical oligonucleotide, confirming that the channel is highly selectivity for oligonucleotide.
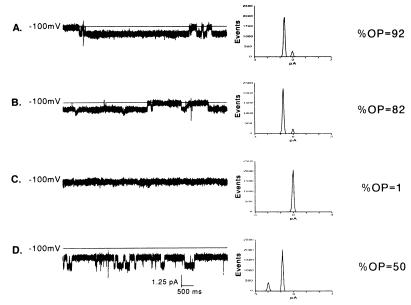
Because oligonucleotides are known to bind to heparin-binding proteins and heparin can block oligonucleotide uptake in some cells (19), we tested whether heparan sulfate could inhibit channel activity. Fig. 4 summarizes a typical experiment by using increasing amounts of heparan sulfate and shows typical current traces, histograms of the distribution of current, and open probability under each condition. Open probability was calculated from 1-min current traces and factored for the number of channels present in the bilayer. As seen in Fig. 4A, open probability of the channel in symmetrical buffered solutions containing 5 μM S-Oligo was 0.92. Addition of heparan sulfate resulted in a dose-dependent reduction in open probability to 0.82 and 0.01 with heparan sulfate at 10 μg/ml and 20 μg/ml, respectively. When heparan sulfate was washed from the bilayer system open probability increased to 0.50 (Fig. 4D).
Figure 4.
Blockade of channel activity with the polyanion heparan sulfate. Continuous 10-s traces (Left), histograms of the distribution of current (Middle), and open probability (Right) in which two doses of heparan sulfate were used to block channel activity. Traces were obtained at a holding potential of −100 mV in an experimental buffer containing 200 mM CsCl and 5 μM S-Oligo (pH 7.4). Heparan sulfate produced a dose-dependent decrease in open probability. Addition of heparan sulfate at 10 μg/ml to both solution chambers decreased open probability from 0.92 (A) to 0.82 (B). Channel activity was completely blocked when heparan sulfate was increased in both chambers to 20 μg/ml (C). Channel activity was restored when heparan sulfate was washed from both chambers (D).
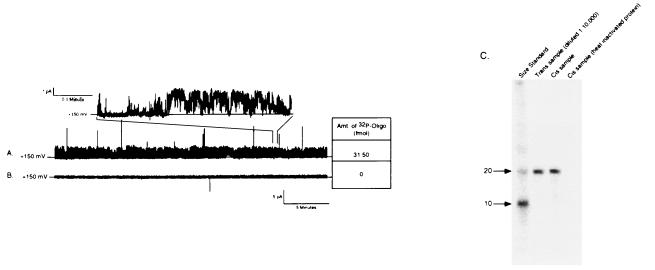
To explore more directly whether oligonucleotides transverse the bilayer via the channel, we radiolabeled oligonucleotides with 32P and measured their movement across the bilayer. After establishing channel activity with symmetrical S-Oligo, [32P]S-Oligo was added to only one chamber (input chamber) to a concentration of 20 nM (approximately 3 × 107 cpm). When channel activity was documented for a minimum of 20 min, samples were collected from both chambers and analyzed by liquid scintillation to quantify oligonucleotide concentration and by gel electrophoresis to confirm that the label had not dissociated from nucleic acids and that the DNA was not degraded. In six experiments under these conditions, channel activity was present for 20–40 min and radioactivity was detected in samples from the chamber opposite to which labeled S-Oligo had been added (collection chamber). Oligonucleotide concentration in the collection chamber solution ranged from 0.5 to 32 fmol/ml. A representative experiment is shown in Fig. 5A. In this example, the bilayer was stable, the channel was active for more than 30 min with a high open probability, and clear channel gating was observed (Fig. 5A, Inset). During this collection interval, 32 fmol of [32P]S-Oligo were detected in the collection chamber. In contrast, when NAC was denatured by boiling prior to forming PLs, channel activity was not observed and [32P]S-Oligo was not detected in the collection chamber (Fig. 5B). When aliquots from these experiments were analyzed by gel electrophoresis (equal counts from each chamber were loaded on the gel), full-length [32P]S-Oligo was detected in samples from the collection chamber (Fig. 5C). Radioisotope was never detected in the collection chamber in the following control experiments: (i) when NAC-PL was not added to the lipid bilayer, (ii) when NAC was present but no channel activity was observed, (iii) when NAC was heat-inactivated prior to forming PLs, (iv) when PLs were formed with active recombinant urate transporter/channel (21), and (v) when PLs were formed with serum albumin. Identical experiments using [32P]Oligo documented movement of native phosphodiester oligonucleotides across the bilayer (data not shown). These data are consistent with the electrophysiologic data and confirm that full-length oligonucleotides moved through the channel and accounted for the current seen under these conditions.
Figure 5.
Direct measurement of oligonucleotide movement across the lipid bilayer. Movement of oligonucleotide across the bilayer was confirmed by tracking the movement of [32P]S-Oligo. [32P]S-Oligo was added to the trans buffer chamber (input chamber) and 20–40 min later samples were collect from both buffer chambers. Radioactivity in the samples was measured by liquid scintillation and analyzed by gel electrophoresis. (A) Representative 30-min current trace obtained at a holding potential of +150 mV. The channel was highly active with clear transition between open and closed states (Inset). During the 30-min collection period approximately 32 fmol of [32P]S-Oligo moved across the lipid membrane. (B) A 30-min current trace obtained from an experiment in which NAC was heat-denatured before forming PLs. Channel activity was not observed and [32P]S-Oligo was not detected in the collection chamber, indicating that oligonucleotide did not move across the bilayer when NAC was rendered inactive. (C) A representative autoradiogram from an experiment to confirm that full-length oligonucleotide moved across the membrane. Samples from the trans (input) and cis (collection) chambers were loaded on a 15% polyacrylamide/7 M urea gel. Before loading, radioactivity in the samples was measured and appropriately diluted to load approximately the same number of counts in each well. Lanes: 1, radiolabeled size markers; 2, sample from the input chamber (diluted 1:10,000 with deionized H2O); 3, sample from collection chamber after 35 min of channel activity; 4, sample from collection chamber obtained 1 h after adding heat-inactivated NAC. In the presence of active NAC, a single band comigrated with the 20-base size marker and the input chamber aliquot. There was no degradation of oligonucleotide as it crossed the bilayer and there was no free 32P detected in the collection chamber solution. Identical results were observed when experiments were performed with radiolabeled Oligo (data not shown).
DISCUSSION
The presence of a nucleic acid receptor and/or channel has been implied for many years. For example, when foreign DNA is injected into mice, antibodies are generated that cross-react with host DNA (1), suggesting that DNA is internalized and presented for antibody generation. Bacterial DNA stimulates in vitro proliferation of T cell-depleted murine lymphocytes (2), suggesting that DNA can trigger B cell proliferation directly through a cell surface-mediated event. Both bacterially derived DNA and antisense oligonucleotides have been shown to induce macrophages to produce interleukin 12 and tumor necrosis factor α (22, 23). All of these effects are thought to involve cell surface receptors or transport proteins that recognize DNA.
Recent advances in the field of antisense oligonucleotide therapy have been particularly suggestive of a DNA receptor/transport mechanism. Studies in which antisense oligonucleotides have been used to inhibit viral genes (24–28) or as therapeutic agents for the treatment of cancer (29, 30) have demonstrated both the utility of antisense as drugs and the importance of DNA binding proteins that mediate internalization. Several potential cell surface DNA binding proteins have been identified (7, 9, 10, 13, 17, 18), but a clear demonstration of function has been difficult, due to limitations of cell culture systems and the difficulty of distinguishing adherent from internalized DNA (15). Recently, however, Benimetskaya et al. (19) have identified a cell surface protein, Mac-1 (CD11b/CD18), that binds oligonucleotides and may, in part, mediate their internalization. Mac-1 is a heparin-binding integrin found primarily on polymorphonuclear leukocytes, macrophages, and natural killer cells. When Mac-1 expression is increased, there is an increase in cell-associated oligonucleotides. Furthermore, monoclonal antibodies directed to either the αM or the β2 subunits of Mac-1 decrease oligonucleotide uptake (19).
Other potential DNA entry mechanisms have been characterized as well, including receptor-mediated endocytosis (12), receptor-mediated but endocytosis-independent uptake (31), and fluid-phase pinocytosis (15). The presence of a specific DNA receptor and/or channel is implied both by the endocytotic pathway and by the receptor-mediated endocytosis-independent pathway. The results from these studies suggest that a protein found on the renal brush border is a highly specific nucleic acid channel clearly different from Mac-1 and could represent a component of the endocytosis-independent pathway.
The presence of a macromolecular channel for DNA, although surprising, has precedence in other cellular systems; several other macromolecule-conducting channels have been described. Proteins have been shown to move into the endoplasmic reticulum through membrane channels (32, 33), current through nuclear pore complexes is reduced during translocation of transcription factors (34, 35), and recently, Kasianowicz et al. (36) showed that DNA (≈150 bases), although it is not the usual substrate, can transverse planar lipid bilayers through α-hemolysin.
In summary, we have purified a highly specific cell surface nucleic acid channel and have demonstrated that both unmodified and sulfur-substituted oligonucleotides pass through this channel. A number of additional questions about the nucleic acid channel remain unanswered by these studies, including the relationship of this channel to the other forms of DNA uptake. Perhaps the most intriguing question is what is the endogenous function of a cell surface nucleic acid channel?
Figure 3.
Summary of channel activity in the presence of a 1,000-fold concentration gradient for S-Oligo. (A) Representative 5-s traces from an experiment obtained at different holding potentials in the presence of a 1,000-fold S-Oligo gradient (500 μM S-Oligo cis and 0.5 μM S-Oligo trans). The solid horizontal line in each trace indicates the closed state. (B) Current–voltage relationship summarizing five experiments in which the presence of an oligonucleotide current was measured after establishing a 1,000-fold concentration gradient for S-Oligo. Conductance of the channel was 10.2 ± 0.7 pS and there was a substantial shift in reversal potential to +28.5 ± 2.8 mV. This shift in reversal potential is consistent with movement of oligonucleotide through the channel.
Acknowledgments
We thank Ruth G. Abramson, M.D., for generous gift of recombinant urate channel protein. We also thank Drs. Mary E. Klotman, Ruth G. Abramson, Jay Rappaport, and Diomedes Logothetis for numerous discussions concerning our data and for critical evaluation of this manuscript. This research was supported in part by the National Cancer Institute, Department of Health and Human Services, with Advanced BioScience Laboratories. B.H. was supported by grants from the National Kidney Foundation of New York and New Jersey and National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health (1PO1DK50795-02). E.L.-P., L.A.B., and P.E.K. were supported by a grant from National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health (1PO1DK50795-02).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: S-Oligo, phosphorothioate antisense oligonucleotide; NAC, nucleic acid binding/channel protein; PL, proteoliposome.
References
- 1.Gilkeson G S, Bernstein K, Pippen A M, Clarke S H, Marion T, Pisetsky D S, Ruiz P, Lefkowith J B. Clin Immunol Immunopathol. 1995;76:59–67. doi: 10.1006/clin.1995.1088. [DOI] [PubMed] [Google Scholar]
- 2.Messina J P, Gilkeson G S, Pisetsky D S. Cell Immunol. 1993;147:148–157. doi: 10.1006/cimm.1993.1055. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal S, Temsamani J, Tang J Y. Proc Natl Acad Sci USA. 1991;88:7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodarzi G, Watabe M, Watabe K. Biopharm Drug Dispos. 1992;13:221–227. doi: 10.1002/bdd.2510130308. [DOI] [PubMed] [Google Scholar]
- 5.Cossum P A, Sasmor H, Dellinger D, Truong L, Cummins L, Owens S R, Markham P M, Shea J P, Crooke S. J Pharmacol Exp Ther. 1993;267:1181–1190. [PubMed] [Google Scholar]
- 6.Oberbauer R, Schreiner G F, Meyer T W. Kidney Int. 1995;48:1226–1232. doi: 10.1038/ki.1995.406. [DOI] [PubMed] [Google Scholar]
- 7.Rappaport J, Hanss B, Kopp J B, Copeland T D, Bruggeman L A, Coffman T M, Klotman P E. Kidney Int. 1995;47:1462–1469. doi: 10.1038/ki.1995.205. [DOI] [PubMed] [Google Scholar]
- 8.Bennett R M, Gabor G T, Merritt M M. J Clin Invest. 1985;76:2182–2190. doi: 10.1172/JCI112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loke S L, Stein C A, Zhang X H, Mori K, Nakanishi M, Subasinghe C, Cohen J S, Neckers L M. Proc Natl Acad Sci USA. 1989;86:3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakubov L A, Deeva E A, Zarytova V F, Ivanova E M, Ryte A S, Yurchenko L V, Vlassov V V. Proc Natl Acad Sci USA. 1989;86:6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iversen P L, Zhu S, Meyer A, Zon G. Antisense Res Dev. 1992;2:211–222. doi: 10.1089/ard.1992.2.211. [DOI] [PubMed] [Google Scholar]
- 12.Wu-Pong S, Weiss T L, Hunt C A. Pharmacol Res. 1992;9:1010–1017. doi: 10.1023/a:1015846209681. [DOI] [PubMed] [Google Scholar]
- 13.Chan T M, Frampton G, Cameron J S. Clin Exp Immunol. 1993;91:110–114. doi: 10.1111/j.1365-2249.1993.tb03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao W Y, Storm C, Egan W, Cheng Y C. Mol Pharmacol. 1993;43:45–50. [PubMed] [Google Scholar]
- 15.Stein C A, Tonkinson J L, Zhang L M, Yakubov L, Gervasoni J, Taub R, Rotenberg S A. Biochemistry. 1993;32:4855–4861. doi: 10.1021/bi00069a022. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q, Matson S, Herrera C J, Fisher E, Yu H, Krieg A M. Antisense Res Dev. 1993;3:53–66. doi: 10.1089/ard.1993.3.53. [DOI] [PubMed] [Google Scholar]
- 17.Geselowitz D A, Neckers L M. Antisense Res Dev. 1992;2:17–25. doi: 10.1089/ard.1992.2.17. [DOI] [PubMed] [Google Scholar]
- 18.Bennett C F, Condon T P, Grimm S, Chan H, Chiang M Y. J Immunol. 1994;152:3530–3540. [PubMed] [Google Scholar]
- 19.Benimetskaya L, Loike J D, Khaled Z, Loike G, Silverstein S C, Cao L, el Khoury J, Cai T, Stein C A. Nat Med. 1997;3:414–420. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- 20.Leal-Pinto E, London R D, Knorr B A, Abramson R G. J Membr Biol. 1995;146:123–132. doi: 10.1007/BF00238003. [DOI] [PubMed] [Google Scholar]
- 21.Leal-Pinto E, Tao W, Rappaport J, Richardson M, Knorr B A, Abramson R G. J Biol Chem. 1997;272:617–625. doi: 10.1074/jbc.272.1.617. [DOI] [PubMed] [Google Scholar]
- 22.Branda R F, Moore A L, Mathews L, McCormack J J, Zon G. Biochem Pharmacol. 1993;45:2037–2043. doi: 10.1016/0006-2952(93)90014-n. [DOI] [PubMed] [Google Scholar]
- 23.Halpern M D, Kurlander R J, Pisetsky D S. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 24.Matsukura M, Zon G, Shinozuka K, Robert-Guroff M, Shimada T, Stein C A, Mitsuya H, Wong-Staal F, Cohen J S, Broder S. Proc Natl Acad Sci USA. 1989;86:4244–4248. doi: 10.1073/pnas.86.11.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao W Y, Hanes R N, Vazquez-Padua M A, Stein C A, Cohen J S, Cheng Y C. Antimicrob Agents Chemother. 1990;34:808–812. doi: 10.1128/aac.34.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Lisziewicz J, Sun D, Zon G, Daefler S, Wong-Staal F, Gallo R C, Klotman M E. J Virol. 1993;67:6882–6888. doi: 10.1128/jvi.67.11.6882-6888.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth G, Curiel T, Lacy J. Blood. 1994;84:582–587. [PubMed] [Google Scholar]
- 28.Tung F Y. J Med Virol. 1994;42:255–258. doi: 10.1002/jmv.1890420310. [DOI] [PubMed] [Google Scholar]
- 29.Higgins K A, Perez J R, Coleman T A, Dorshkind K, McComas W A, Sarmiento U M, Rosen C A, Narayanan R. Proc Natl Acad Sci USA. 1993;90:9901–9905. doi: 10.1073/pnas.90.21.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resnicoff M, Sell C, Rubini M, Coppola D, Ambrose D, Baserga R, Rubin R. Cancer Res. 1994;54:2218–2222. [PubMed] [Google Scholar]
- 31.Wu-Pong S, Weiss T L, Hunt C A. Cell Mol Biol. 1994;40:843–850. [PubMed] [Google Scholar]
- 32.Simon S M, Blobel G. Cell. 1991;65:371–380. doi: 10.1016/0092-8674(91)90455-8. [DOI] [PubMed] [Google Scholar]
- 33.Simon S M, Blobel G. Cell. 1992;69:677–684. doi: 10.1016/0092-8674(92)90231-z. [DOI] [PubMed] [Google Scholar]
- 34.Bustamante J O, Oberleithner H, Hanover J A, Liepins A. J Membr Biol. 1995;146:253–261. doi: 10.1007/BF00233945. [DOI] [PubMed] [Google Scholar]
- 35.Bustamante J O, Hanover J A, Liepins A. J Membr Biol. 1995;146:239–251. doi: 10.1007/BF00233944. [DOI] [PubMed] [Google Scholar]
- 36.Kasianowicz J J, Brandin E, Branton D, Deamer D W. Proc Natl Acad Sci USA. 1996;93:13770–13773. doi: 10.1073/pnas.93.24.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]