Abstract
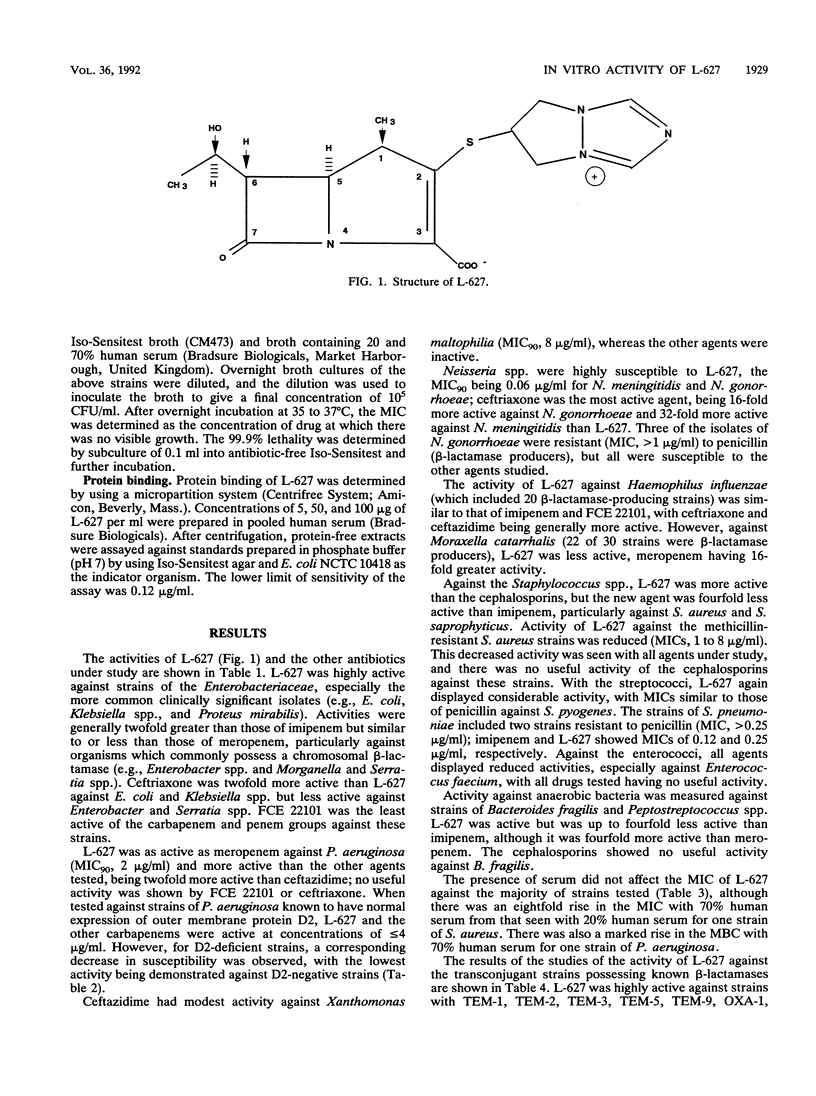
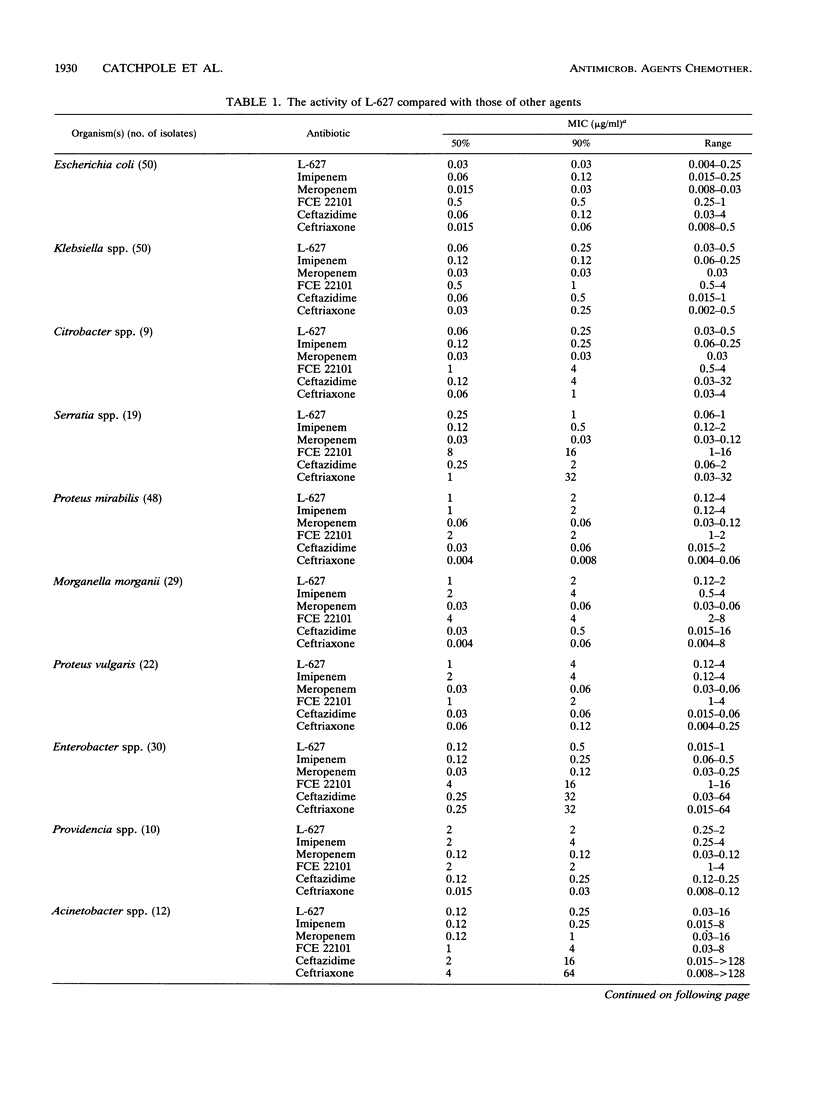
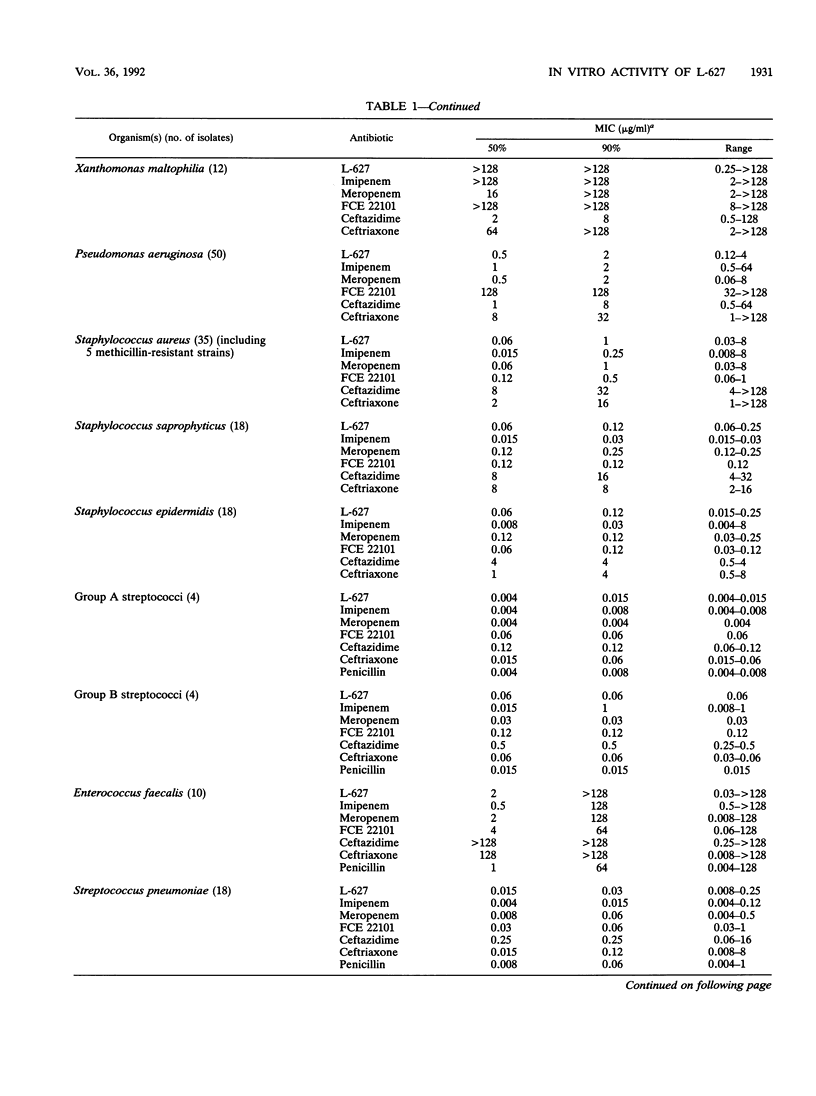
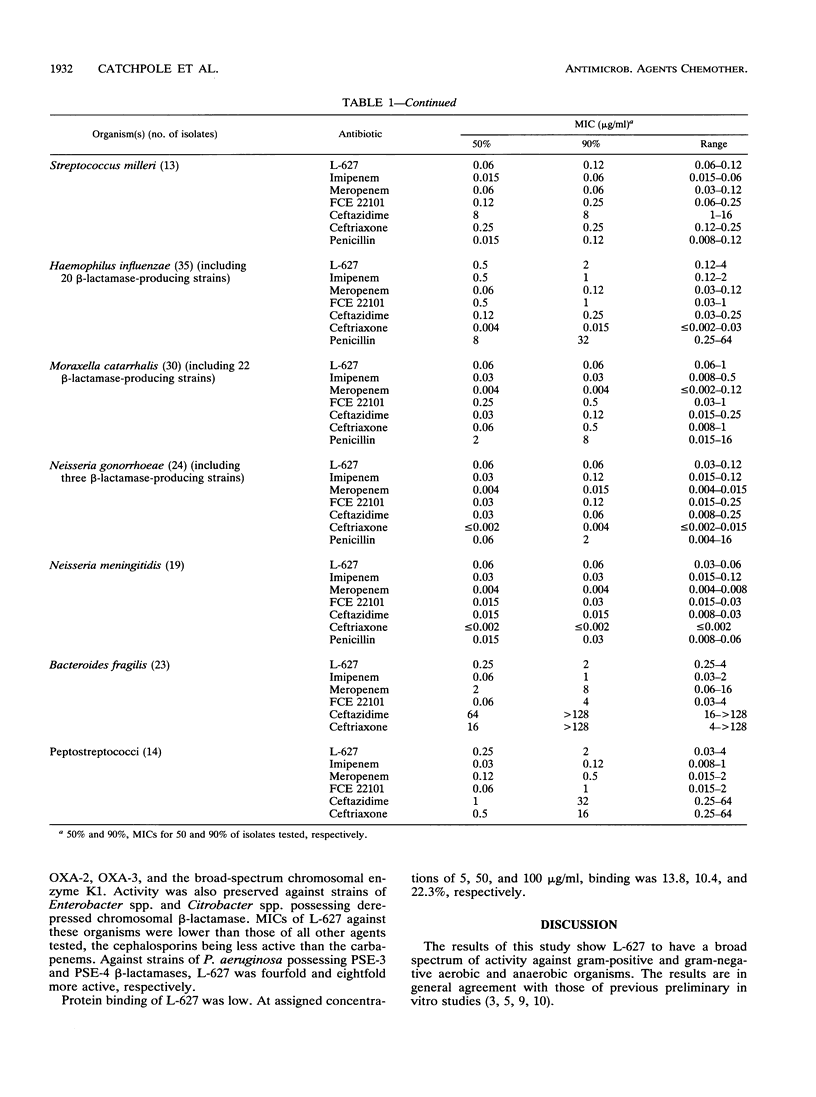
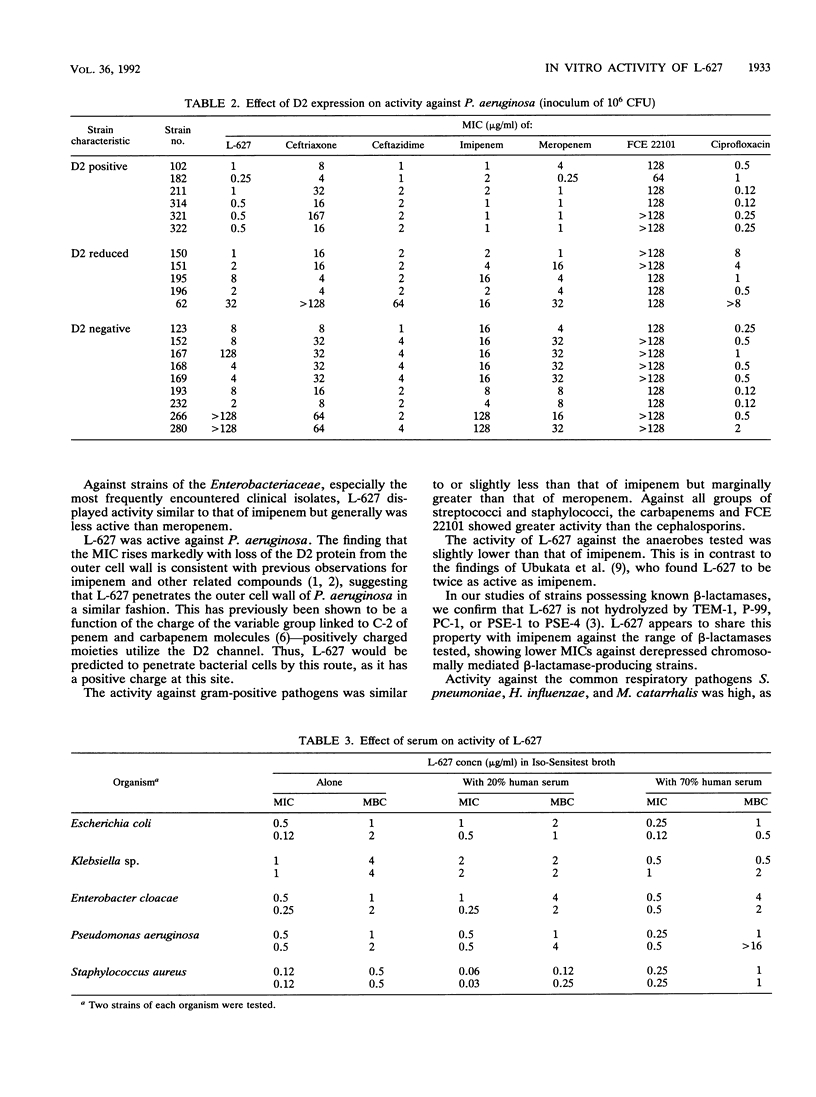
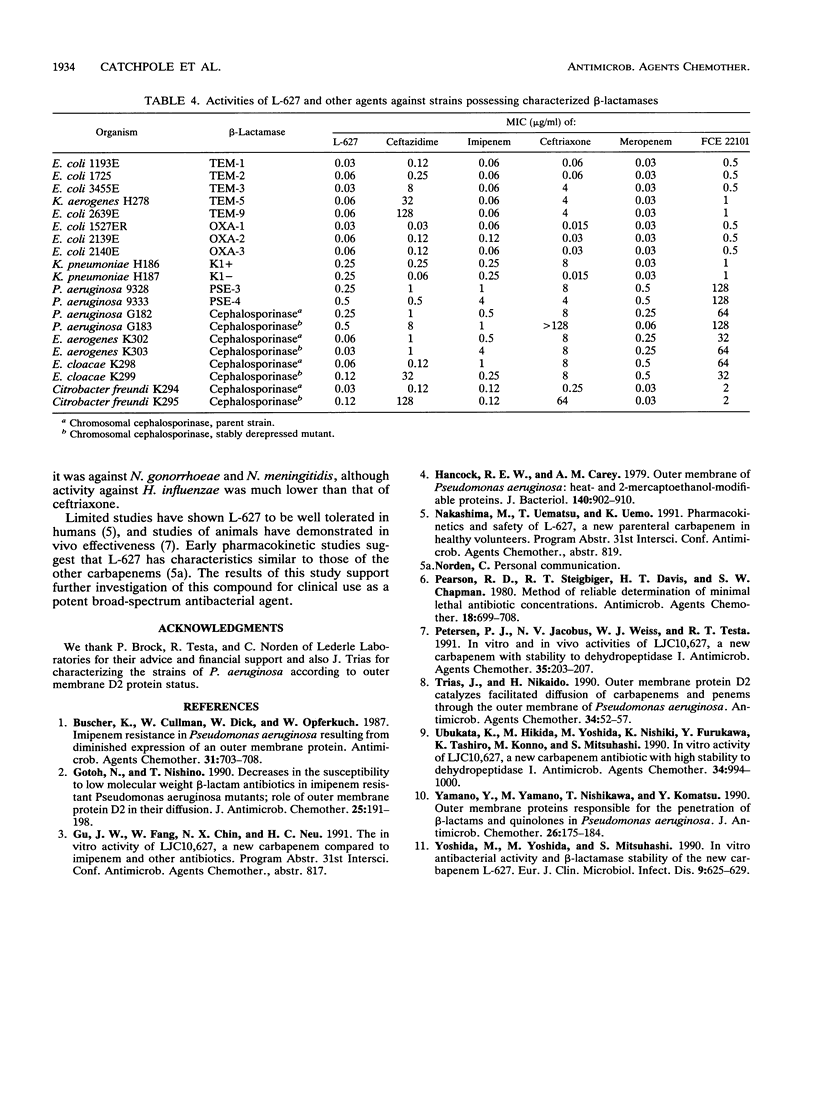
The in vitro activity of L-627, a new parenterally administered carbapenem, was compared with those of imipenem, meropenem, FCE 22101 (a penem), ceftazidime, and ceftriaxone. L-627 was active against members of the family Enterobacteriaceae (MIC for 90% of strains tested [MIC90] ranging from 0.03 to 4 micrograms/ml). L-627 displayed activity equal to that of meropenem against Pseudomonas aeruginosa (MIC90, 2 micrograms/ml), although, as with other carbapenems, the antipseudomonal activity was reduced against D2-deficient strains. Staphylococci and streptococci were susceptible (MIC90 of 1.0 micrograms/ml for Staphylococcus aureus and 0.015 micrograms/ml for group A streptococci). L-627 also had activity against anaerobic bacteria (MIC90, 2.0 micrograms/ml for Bacteroides fragilis). Neisseria gonorrhoeae and Neisseria meningitidis were highly susceptible (MIC90, 0.06 micrograms/ml), and against the common respiratory pathogens (Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis), the MIC90s were less than or equal to 2.0 micrograms/ml. The protein binding of L-627 ranged from 13.8 to 22%, depending on the concentration. The presence of human serum had little effect on the MIC or MBC of L-627. These results suggest that L-627 merits further study in the treatment of infections caused by a wide range of pathogens.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büscher K. H., Cullmann W., Dick W., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob Agents Chemother. 1987 May;31(5):703–708. doi: 10.1128/aac.31.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Nishino T. Decreases of the susceptibility to low molecular weight beta-lactam antibiotics in imipenem-resistant Pseudomonas aeruginosa mutants: role of outer membrane protein D2 in their diffusion. J Antimicrob Chemother. 1990 Feb;25(2):191–198. doi: 10.1093/jac/25.2.191. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen P. J., Jacobus N. V., Weiss W. J., Testa R. T. In vitro and in vivo activities of LJC10,627, a new carbapenem with stability to dehydropeptidase I. Antimicrob Agents Chemother. 1991 Jan;35(1):203–207. doi: 10.1128/aac.35.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jan;34(1):52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Hikida M., Yoshida M., Nishiki K., Furukawa Y., Tashiro K., Konno M., Mitsuhashi S. In vitro activity of LJC10,627, a new carbapenem antibiotic with high stability to dehydropeptidase I. Antimicrob Agents Chemother. 1990 Jun;34(6):994–1000. doi: 10.1128/aac.34.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano Y., Nishikawa T., Komatsu Y. Outer membrane proteins responsible for the penetration of beta-lactams and quinolones in Pseudomonas aeruginosa. J Antimicrob Chemother. 1990 Aug;26(2):175–184. doi: 10.1093/jac/26.2.175. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Mitsuhashi S. In vitro antibacterial activity and beta-lactamase stability of the new carbapenem LJC10,627. Eur J Clin Microbiol Infect Dis. 1990 Aug;9(8):625–629. doi: 10.1007/BF01967222. [DOI] [PubMed] [Google Scholar]


