Abstract
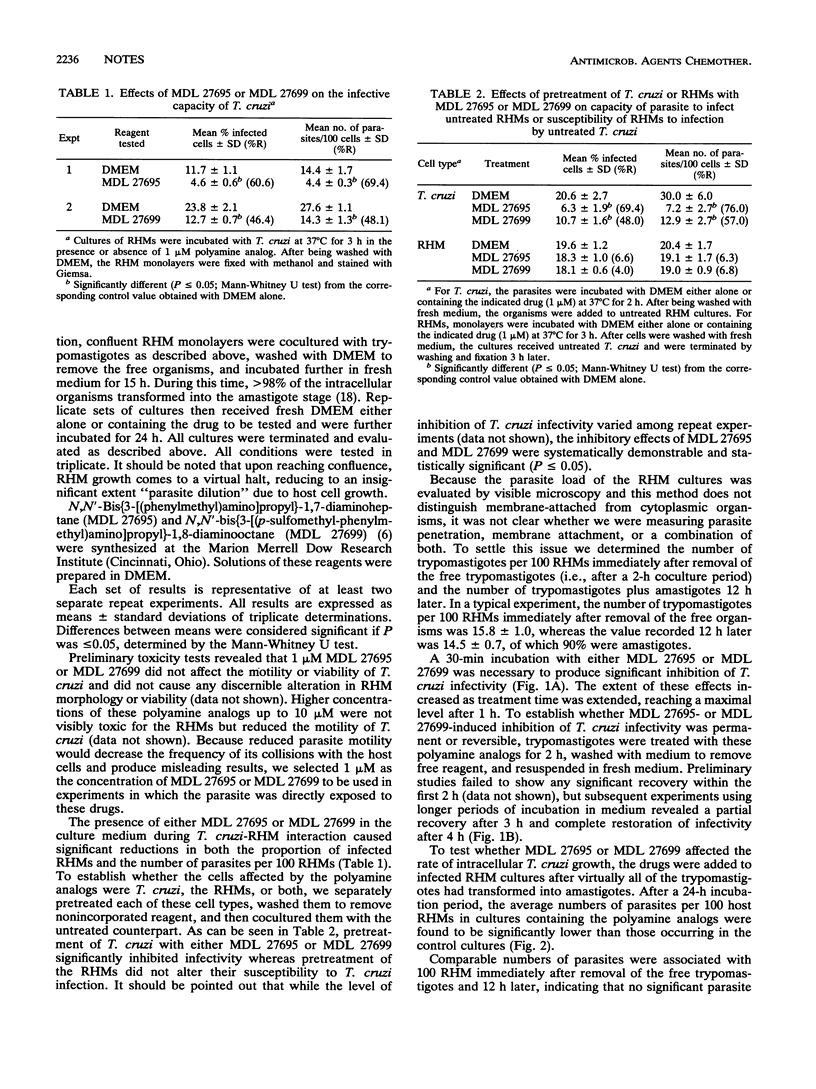
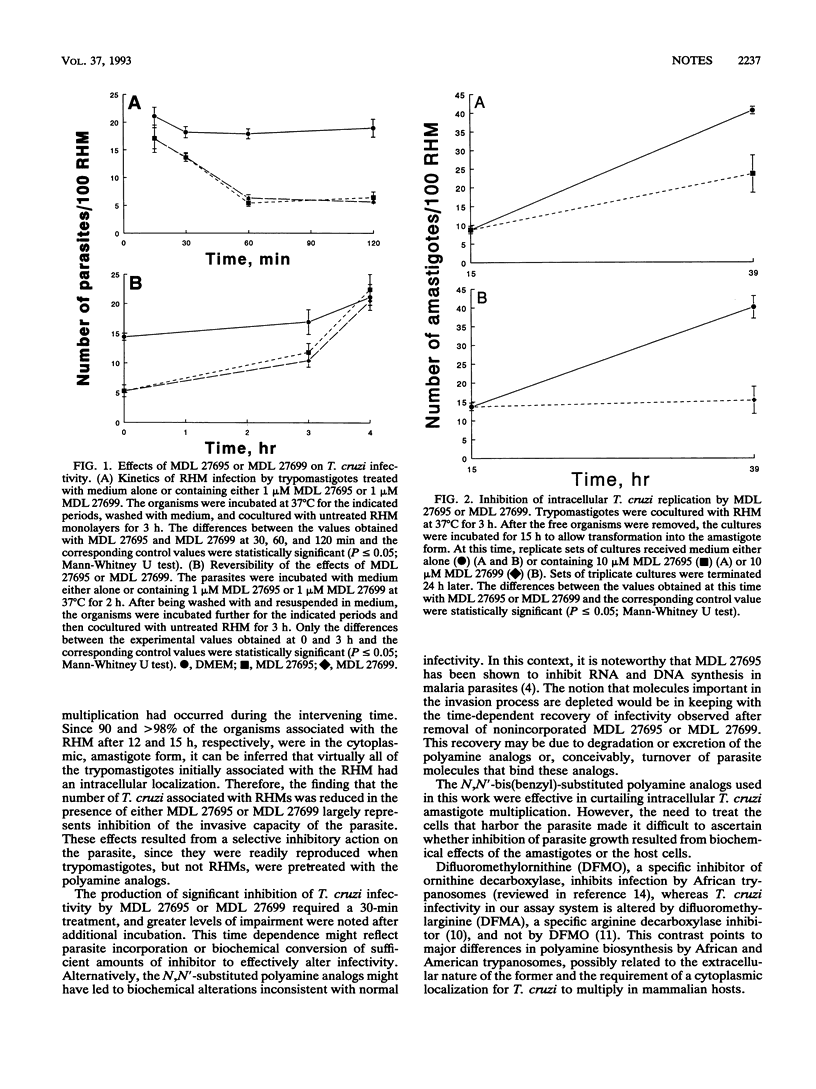
We studied the effects of two N,N'-bis(benzyl)-substituted polyamine analogs on the capacities of Trypanosoma cruzi to invade and multiply within a mammalian host cell. At concentrations as low as 1 microM, these compounds reduced significantly the infectivity of the parasite for rat heart myoblasts in a time-dependent manner. Pretreatment of virulent T. cruzi trypomastigotes, but not myoblast pretreatment, reduced the level of infectivity. The inhibitory effects started to subside 3 h after removal of the drugs and were no longer detectable after 4 h. A significant decrease in the rate of intracellular amastigote multiplication was also seen when the drugs were added to myoblast cultures which had been previously infected with untreated T. cruzi. These results show that N,N'-bis(benzyl)-substituted polyamine analogs meet the two most important criteria for potential chemotherapeutic agents against T. cruzi infection, namely, inhibition of both host cell invasion and intracellular replication by this parasite.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann R. J., Hanson W. L., McCann P. P., Sjoerdsma A., Bitonti A. J. Suppression of both antimony-susceptible and antimony-resistant Leishmania donovani by a bis(benzyl)polyamine analog. Antimicrob Agents Chemother. 1990 May;34(5):722–727. doi: 10.1128/aac.34.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann R. J., McCann P. P., Bitonti A. J. Suppression of Leishmania donovani by oral administration of a bis(benzyl)polyamine analog. Antimicrob Agents Chemother. 1991 Jul;35(7):1403–1407. doi: 10.1128/aac.35.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., Dumont J. A., Bush T. L., Edwards M. L., Stemerick D. M., McCann P. P., Sjoerdsma A. Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with alpha-difluoromethylornithine cure murine malaria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):651–655. doi: 10.1073/pnas.86.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzko D. B., Kierszenbaum F. Isolation of Trypanosoma cruzi from blood. J Parasitol. 1974 Dec;60(6):1037–1038. [PubMed] [Google Scholar]
- Edwards M. L., Stemerick D. M., Bitonti A. J., Dumont J. A., McCann P. P., Bey P., Sjoerdsma A. Antimalarial polyamine analogues. J Med Chem. 1991 Feb;34(2):569–574. doi: 10.1021/jm00106a015. [DOI] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-alpha-(Difluoromethyl)arginine: a potent enzyme-activated irreversible inhibitor of bacterial decarboxylases. Biochemistry. 1981 May 26;20(11):3163–3168. doi: 10.1021/bi00514a027. [DOI] [PubMed] [Google Scholar]
- Kelly M., McCann P. P., Schindler J. Alterations in polyamine metabolism during embryonal carcinoma cell differentiation in vitro. Dev Biol. 1985 Oct;111(2):510–514. doi: 10.1016/0012-1606(85)90502-0. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Wirth J. J., McCann P. P., Sjoerdsma A. Arginine decarboxylase inhibitors reduce the capacity of Trypanosoma cruzi to infect and multiply in mammalian host cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4278–4282. doi: 10.1073/pnas.84.12.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Wirth J. J., McCann P. P., Sjoerdsma A. Impairment of macrophage function by inhibitors of ornithine decarboxylase activity. Infect Immun. 1987 Oct;55(10):2461–2464. doi: 10.1128/iai.55.10.2461-2464.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M. F., Kierszenbaum F. Effects of treatment of trypomastigote forms of Trypanosoma cruzi or host cells with ethidium bromide on cell infection and intracellular fate of the parasite. J Parasitol. 1984 Dec;70(6):911–917. [PubMed] [Google Scholar]
- Majumder S., Wirth J. J., Bitonti A. J., McCann P. P., Kierszenbaum F. Biochemical evidence for the presence of arginine decarboxylase activity in Trypanosoma cruzi. J Parasitol. 1992 Apr;78(2):371–374. [PubMed] [Google Scholar]
- Mercado T. I., Katusha K. Isolation of Trypanosoma cruzi from the blood of infected mice by column chromatography. Prep Biochem. 1979;9(1):97–106. doi: 10.1080/00327487908061675. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Fibronectin enhances macrophage association with invasive forms of Trypanosoma cruzi. J Immunol. 1984 Jul;133(1):460–464. [PubMed] [Google Scholar]
- Yakubu M. A., Basso B., Kierszenbaum F. DL-alpha-difluoromethylarginine inhibits intracellular Trypanosoma cruzi multiplication by affecting cell division but not trypomastigote-amastigote transformation. J Parasitol. 1992 Jun;78(3):414–419. [PubMed] [Google Scholar]


