Abstract
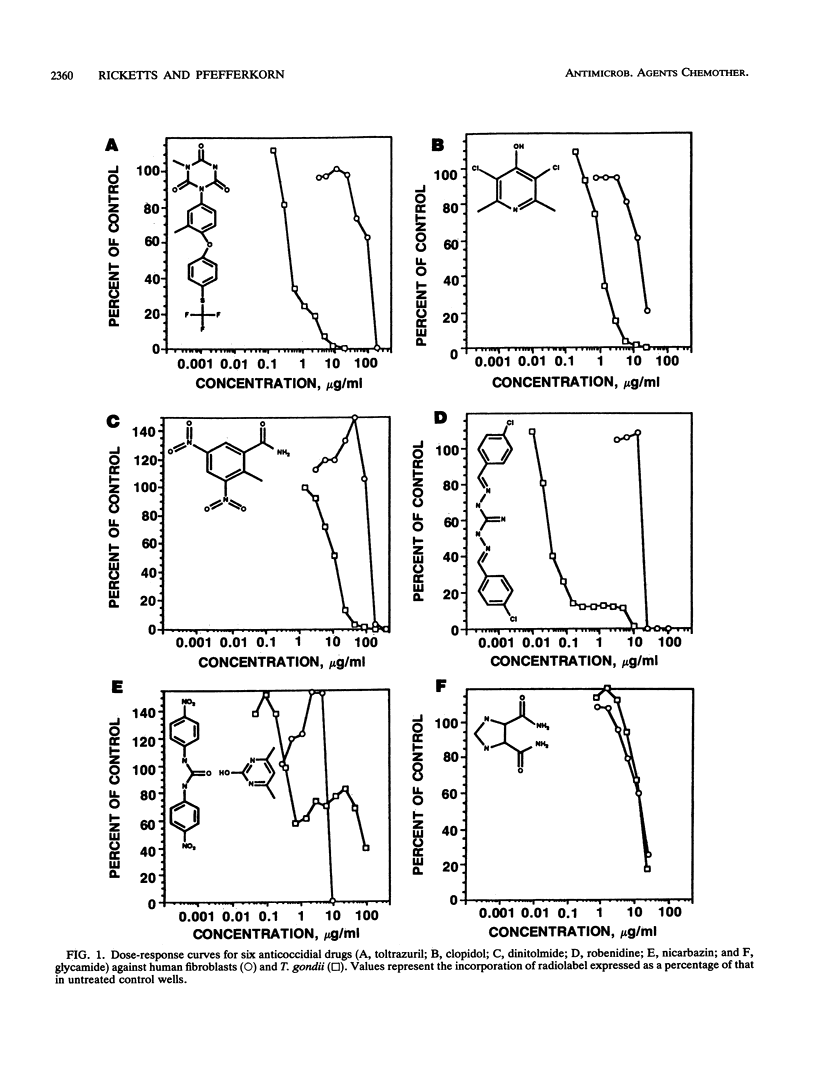
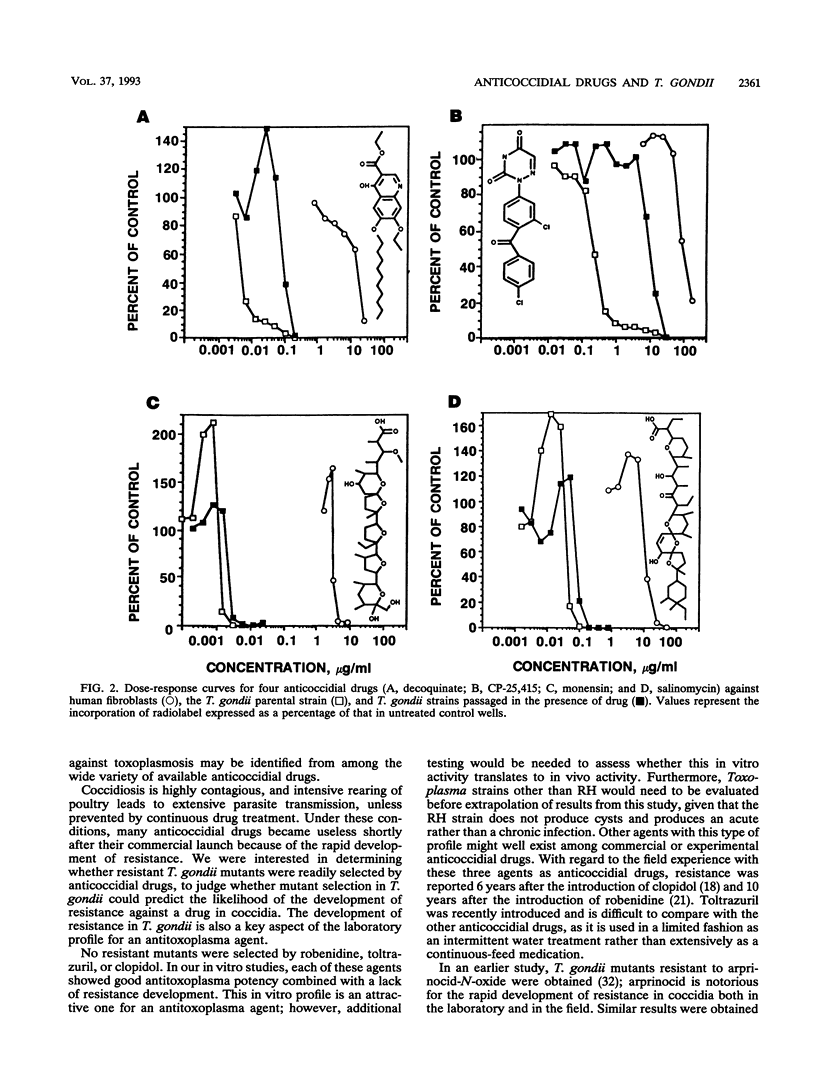
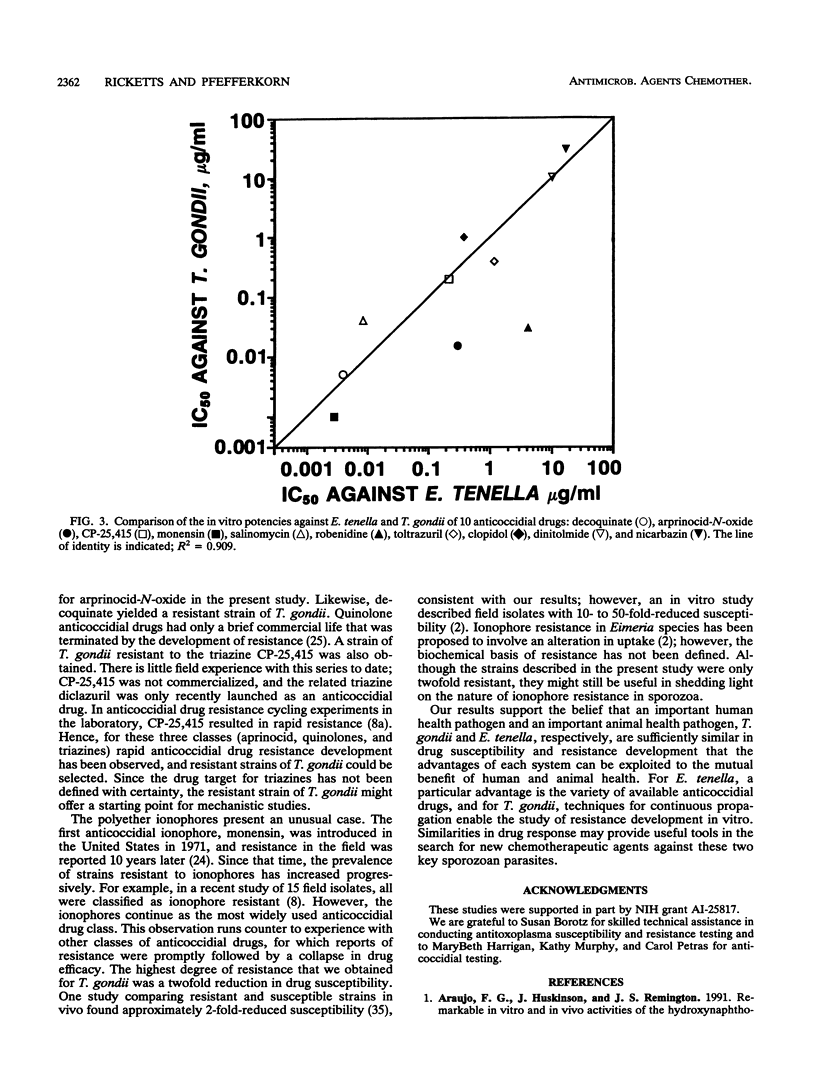
Anticoccidial drugs were evaluated for activity and for the development of resistance in a model of Toxoplasma gondii growing in human fibroblast cultures. Of 13 anticoccidial drugs tested, 9 had selective antitoxoplasma activity (50% inhibitory concentration, in micrograms per milliliter): decoquinate (0.005), arprinocid-N-oxide (0.015), robenidine (0.03), the aryl triazine CP-25,415 (0.2), toltrazuril (0.4), clopidol (1), dinitolmide (Zoalene; Dow) (10), and the carboxylic acid ionophores monensin (0.001) and salinomycin (0.04). Glycarbylamide, amprolium, nicarbazin, and the 6-(p-bromophenoxy)-7-chloro analog of halofuginone (Stenorol; Roussel-UCLAF) (CP-63,567) were toxic for the fibroblasts. Since Eimeria tenella has a similar drug susceptibility profile, anticoccidial durgs can be viewed as a potential source of new antitoxoplasma therapies. The development of resistance has limited the usefulness of most of these drugs as anticoccidial agents; in coccidia, resistance to all except the ionophores occurs readily in vivo. We explored the development of resistance in T. gondii by attempting to select mutants in vitro from parasites mutagenized with ethylnitrosourea. Mutants that had 20- to 50-fold-reduced susceptibility to decoquinate, arprinocid-N-oxide, and CP-25,415 were obtained. Ionophore-resistant T. gondii mutants were also selected in vitro; however, there was only a twofold difference in susceptibility between these mutants and the wild type. For three drugs (clopidol, robenidine, and toltrazuril), we were unable to select resistant mutants. For experimental anticoccidial drugs, there is currently no in vitro method for assessing the risk of development of resistance in Eimeria species. Our results suggest that T. gondii may offer a useful surrogate for this assessment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Huskinson J., Remington J. S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991 Feb;35(2):293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine P. C., Smith C. K., 2nd, Danforth H. D., Ruff M. D. Effect of ionophorous anticoccidials on invasion and development of Eimeria: comparison of sensitive and resistant isolates and correlation with drug uptake. Poult Sci. 1987 Jun;66(6):960–965. doi: 10.3382/ps.0660960. [DOI] [PubMed] [Google Scholar]
- Bedrník P. Antitoxoplasma activity of coccidiostatics. Folia Parasitol (Praha) 1972;19(2):129–132. [PubMed] [Google Scholar]
- Chang H. R., Comte R., Pechère J. C. In vitro and in vivo effects of doxycycline on Toxoplasma gondii. Antimicrob Agents Chemother. 1990 May;34(5):775–780. doi: 10.1128/aac.34.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. R., Comte R., Piguet P. F., Pechère J. C. Activity of minocycline against Toxoplasma gondii infection in mice. J Antimicrob Chemother. 1991 May;27(5):639–645. doi: 10.1093/jac/27.5.639. [DOI] [PubMed] [Google Scholar]
- Chang H. R., Pechère J. C. In vitro effects of four macrolides (roxithromycin, spiramycin, azithromycin [CP-62,993], and A-56268) on Toxoplasma gondii. Antimicrob Agents Chemother. 1988 Apr;32(4):524–529. doi: 10.1128/aac.32.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappel L. R., Howes H. L., Lynch J. E. The site of action of a broad-spectrum aryltriazine anticoccidial, CP-25,415. J Parasitol. 1974 Jun;60(3):415–420. [PubMed] [Google Scholar]
- Gellin B. G., Soave R. Coccidian infections in AIDS. Toxoplasmosis, cryptosporidiosis, and isosporiasis. Med Clin North Am. 1992 Jan;76(1):205–234. doi: 10.1016/s0025-7125(16)30377-7. [DOI] [PubMed] [Google Scholar]
- Haverkos H. W. Assessment of therapy for toxoplasma encephalitis. The TE Study Group. Am J Med. 1987 May;82(5):907–914. doi: 10.1016/0002-9343(87)90151-3. [DOI] [PubMed] [Google Scholar]
- Hudson A. T., Randall A. W., Fry M., Ginger C. D., Hill B., Latter V. S., McHardy N., Williams R. B. Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoal activity. Parasitology. 1985 Feb;90(Pt 1):45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- Hupe D. J., Pfefferkorn E. R., Behrens N. D., Peters K. L-651,582 inhibition of intracellular parasitic protozoal growth correlates with host-cell directed effects. J Pharmacol Exp Ther. 1991 Feb;256(2):462–467. [PubMed] [Google Scholar]
- Jeffers T. K. Eimeria tenella: incidence, distribution, and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis. 1974 Jan-Mar;18(1):74–84. [PubMed] [Google Scholar]
- Luft B. J., Brooks R. G., Conley F. K., McCabe R. E., Remington J. S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [PubMed] [Google Scholar]
- Mathis G. F., McDougald L. R. Drug responsiveness of field isolates of chicken Coccidia. Poult Sci. 1982 Jan;61(1):38–45. doi: 10.3382/ps.0610038. [DOI] [PubMed] [Google Scholar]
- McCabe R. E., Luft B. J., Remington J. S. Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis. 1984 Dec;150(6):961–962. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- McDougald L. R. Anticoccidial drug resistance in the southeastern United States: polyether, ionophorous drugs. Avian Dis. 1981 Jul-Sep;25(3):600–609. [PubMed] [Google Scholar]
- McManus E. C., Campbell W. C., Cuckler A. C. Development of resistance to quinoline coccidiostats under field and laboratory conditions. J Parasitol. 1968 Dec;54(6):1190–1193. [PubMed] [Google Scholar]
- Melton M. L., Sheffield H. G. Activity of the anticoccidial compound, lasalocid, against Toxoplasma gondii in cultured cells. J Parasitol. 1975 Aug;61(4):713–717. [PubMed] [Google Scholar]
- Millard K. W., Spelman J. S. Alternative to monensin in sheep. Vet Rec. 1989 May 20;124(20):547–547. doi: 10.1136/vr.124.20.547-d. [DOI] [PubMed] [Google Scholar]
- PEARDON D. L., BILKOVICH F. R., TODD A. C., HOYT H. H. TRIALS OF CANDIDATE BOVINE COCCIDIOSTATS: EFFICACY OF AMPROLIUM, LINCOMYCIN, SULFAMETHAZINE, CHLOROQUINE SULFATE, AND DI-PHENTHANE-70. Am J Vet Res. 1965 May;26:683–687. [PubMed] [Google Scholar]
- Patton W. H. Eimeria tenella: cultivation of the asexual stages in cultured animal cells. Science. 1965 Nov 5;150(3697):767–769. doi: 10.1126/science.150.3697.767. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Eckel M. E., McAdams E. Toxoplasma gondii: in vivo and in vitro studies of a mutant resistant to arprinocid-N-oxide. Exp Parasitol. 1988 Apr;65(2):282–289. doi: 10.1016/0014-4894(88)90133-6. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976 Jun;39(3):365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Toxoplasma gondii: the enzymic defect of a mutant resistant to 5-fluorodeoxyuridine. Exp Parasitol. 1978 Feb;44(1):26–35. doi: 10.1016/0014-4894(78)90077-2. [DOI] [PubMed] [Google Scholar]
- Ricketts A. P. Eimeria tenella: growth and drug sensitivity in tissue culture under reduced oxygen. Exp Parasitol. 1992 Jun;74(4):463–469. doi: 10.1016/0014-4894(92)90208-r. [DOI] [PubMed] [Google Scholar]
- Ryley J. F. Drug resistance in coccidia. Adv Vet Sci Comp Med. 1980;24:99–120. [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]


