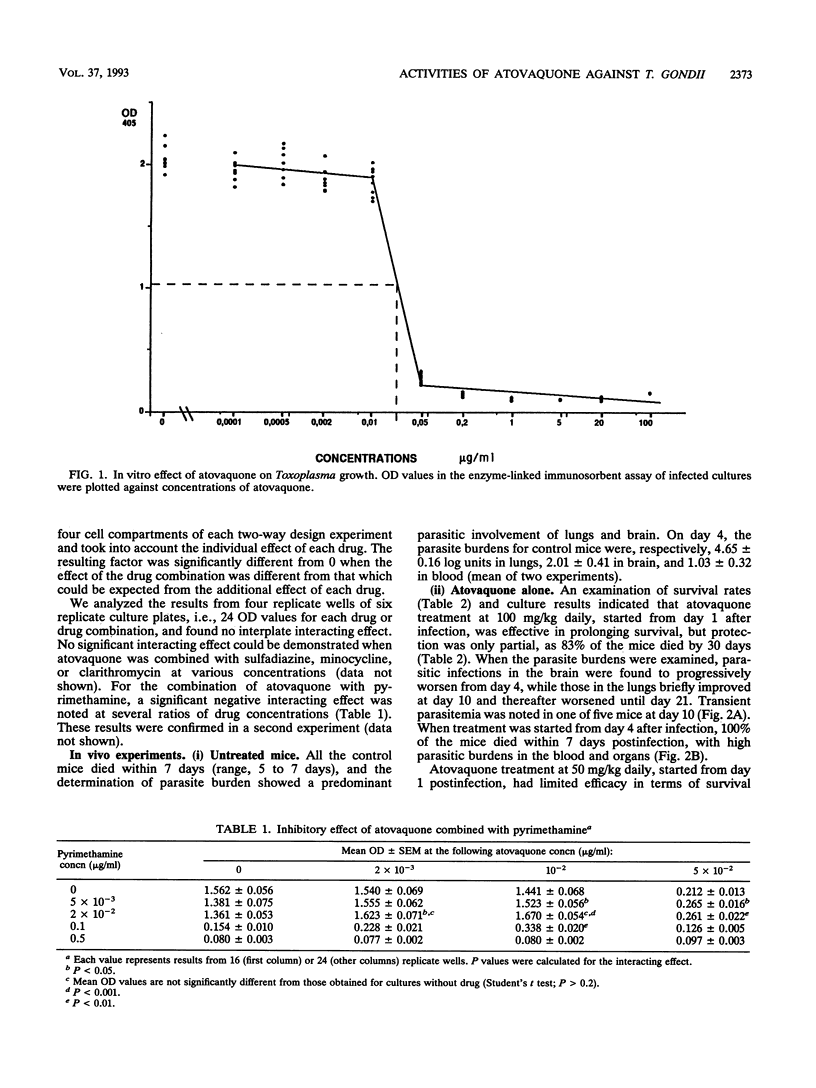
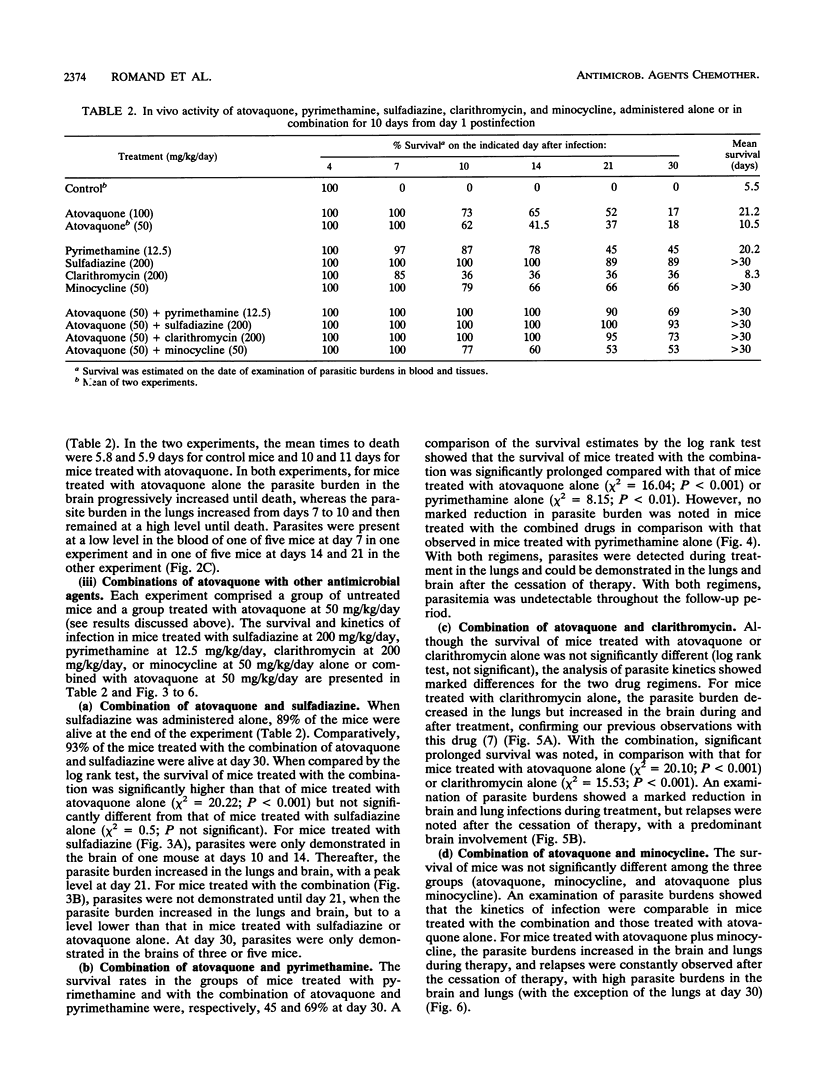
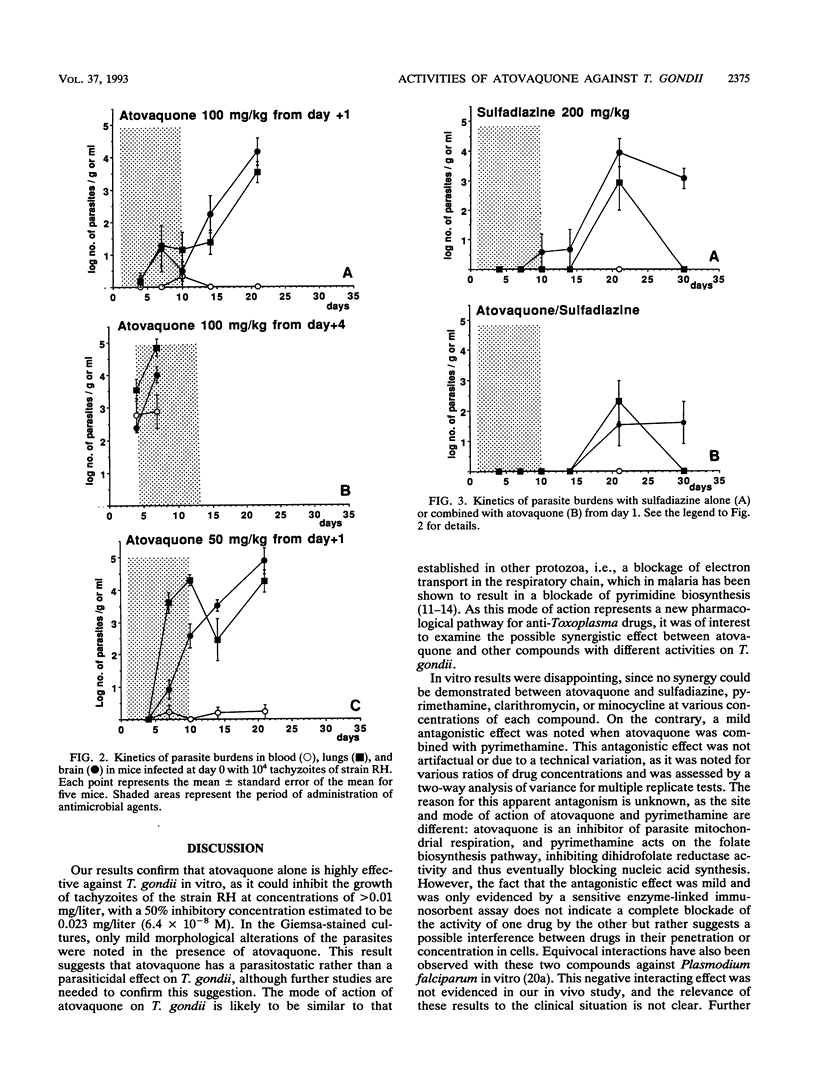
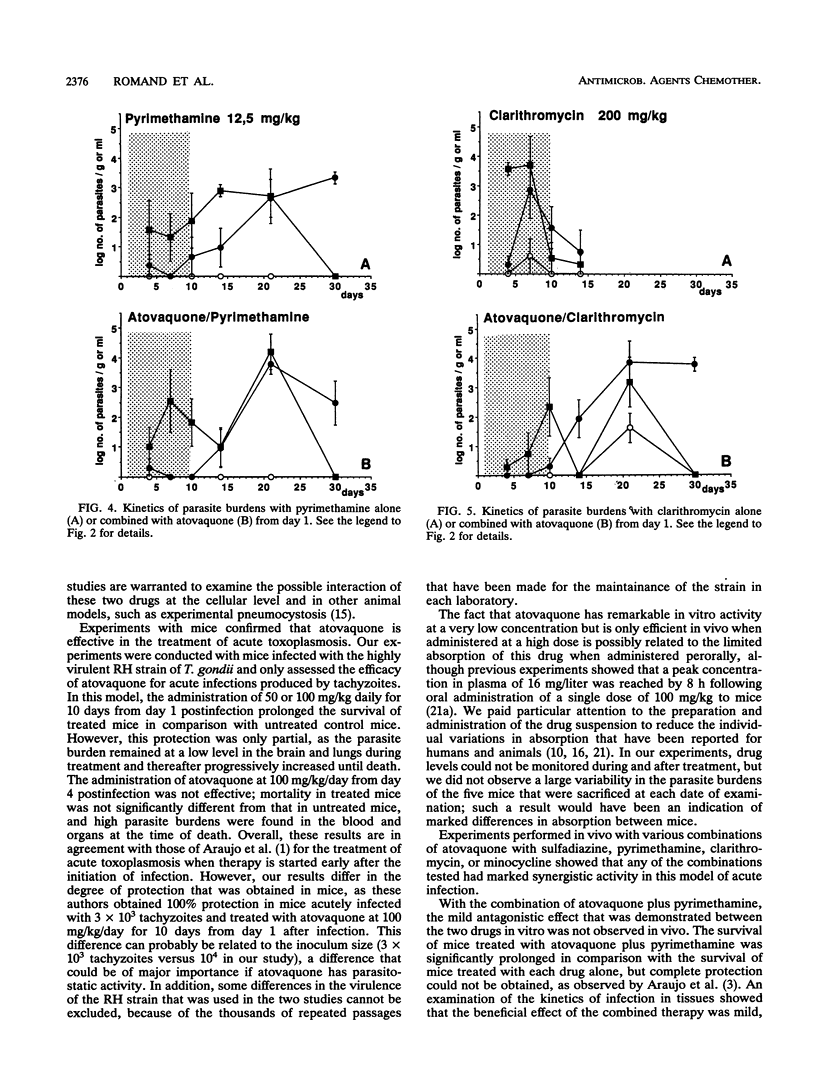
Abstract
The efficacy of atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, and minocycline was examined in vitro and in a murine model of acute toxoplasmosis. In vitro studies were performed with MRC5 fibroblast tissue cultures, with quantification of Toxoplasma growth by an enzyme-linked immunosorbent assay. For in vivo studies, mice were acutely infected intraperitoneally with 10(4) tachyzoites of the virulent RH strain and then treated perorally for 10 days from day 1 postinfection. The following drug regimens were investigated: atovaquone at 100 and 50 mg/kg of body weight per day and the combinations of atovaquone at 50 mg/kg with sulfadiazine at 200 mg/kg, pyrimethamine at 12.5 mg/kg, clarithromycin at 200 mg/kg, or minocycline at 50 mg/kg. Efficacy was assessed by determination of survival rates and sequential determination of parasite burdens in blood, brain, and lungs. In vitro, atovaquone inhibited Toxoplasma growth at a concentration of > or = 0.02 mg/liter; the 50% inhibitory concentration was estimated to be 0.023 mg/liter. No synergistic effect was observed when it was combined with sulfadiazine, clarithromycin, or minocycline, whereas a significant antagonistic effect was noted for the combination of atovaquone with pyrimethamine. In vivo, administration of atovaquone at 100 or 50 mg/kg/day for 10 days resulted in prolonged survival compared with that in untreated mice; this survival was associated with a reduction of parasite burdens in blood and tissues during the course of treatment. The combinations of atovaquone with pyrimethamine, clarithromycin, or sulfadiazine were more efficient than each drug administered alone, in terms of survival, but parasite burdens in blood and organs were not reduced compared with those in mice treated with any of the agents alone. These experimental results confirmed the activity of atovaquone against Toxoplasma gondii, but no marked improvement in efficacy was observed in vitro and in vivo when this drug was combined with pyrimethamine, sulfadiazine, minocycline, or clarithromycin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Huskinson-Mark J., Gutteridge W. E., Remington J. S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992 Feb;36(2):326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Huskinson J., Remington J. S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991 Feb;35(2):293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Lin T., Remington J. S. The activity of atovaquone (566C80) in murine toxoplasmosis is markedly augmented when used in combination with pyrimethamine or sulfadiazine. J Infect Dis. 1993 Feb;167(2):494–497. doi: 10.1093/infdis/167.2.494. [DOI] [PubMed] [Google Scholar]
- Araujo F. G., Prokocimer P., Lin T., Remington J. S. Activity of clarithromycin alone or in combination with other drugs for treatment of murine toxoplasmosis. Antimicrob Agents Chemother. 1992 Nov;36(11):2454–2457. doi: 10.1128/aac.36.11.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Almadany R., Chau F., Rouveix B., Pocidalo J. J. Synergistic activity of azithromycin and pyrimethamine or sulfadiazine in acute experimental toxoplasmosis. Antimicrob Agents Chemother. 1992 May;36(5):997–1001. doi: 10.1128/aac.36.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Caroff B., Chau F., Prokocimer P., Pocidalo J. J. Synergistic activity of clarithromycin and minocycline in an animal model of acute experimental toxoplasmosis. Antimicrob Agents Chemother. 1992 Dec;36(12):2852–2855. doi: 10.1128/aac.36.12.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Chastang C. Activity in vitro against Toxoplasma gondii of azithromycin and clarithromycin alone and with pyrimethamine. J Antimicrob Chemother. 1990 Apr;25(4):708–711. doi: 10.1093/jac/25.4.708. [DOI] [PubMed] [Google Scholar]
- Derouin F., Chastang C. Enzyme immunoassay to assess effect of antimicrobial agents on Toxoplasma gondii in tissue culture. Antimicrob Agents Chemother. 1988 Mar;32(3):303–307. doi: 10.1128/aac.32.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falloon J., Kovacs J., Hughes W., O'Neill D., Polis M., Davey R. T., Jr, Rogers M., LaFon S., Feuerstein I., Lancaster D. A preliminary evaluation of 566C80 for the treatment of Pneumocystis pneumonia in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1991 Nov 28;325(22):1534–1538. doi: 10.1056/NEJM199111283252202. [DOI] [PubMed] [Google Scholar]
- Fry M., Hudson A. T., Randall A. W., Williams R. B. Potent and selective hydroxynaphthoquinone inhibitors of mitochondrial electron transport in Eimeria tenella (Apicomplexa: Coccidia). Biochem Pharmacol. 1984 Jul 1;33(13):2115–2122. doi: 10.1016/0006-2952(84)90581-1. [DOI] [PubMed] [Google Scholar]
- Fry M., Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4'-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem Pharmacol. 1992 Apr 1;43(7):1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- Hudson A. T., Dickins M., Ginger C. D., Gutteridge W. E., Holdich T., Hutchinson D. B., Pudney M., Randall A. W., Latter V. S. 566C80: a potent broad spectrum anti-infective agent with activity against malaria and opportunistic infections in AIDS patients. Drugs Exp Clin Res. 1991;17(9):427–435. [PubMed] [Google Scholar]
- Hughes W. T., Gray V. L., Gutteridge W. E., Latter V. S., Pudney M. Efficacy of a hydroxynaphthoquinone, 566C80, in experimental Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1990 Feb;34(2):225–228. doi: 10.1128/aac.34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., Kennedy W., Shenep J. L., Flynn P. M., Hetherington S. V., Fullen G., Lancaster D. J., Stein D. S., Palte S., Rosenbaum D. Safety and pharmacokinetics of 566C80, a hydroxynaphthoquinone with anti-Pneumocystis carinii activity: a phase I study in human immunodeficiency virus (HIV)-infected men. J Infect Dis. 1991 Apr;163(4):843–848. doi: 10.1093/infdis/163.4.843. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A. Efficacy of atovaquone in treatment of toxoplasmosis in patients with AIDS. The NIAID-Clinical Center Intramural AIDS Program. Lancet. 1992 Sep 12;340(8820):637–638. doi: 10.1016/0140-6736(92)92172-c. [DOI] [PubMed] [Google Scholar]
- Lopez J. S., de Smet M. D., Masur H., Mueller B. U., Pizzo P. A., Nussenblatt R. B. Orally administered 566C80 for treatment of ocular toxoplasmosis in a patient with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1992 Mar 15;113(3):331–333. doi: 10.1016/s0002-9394(14)71588-0. [DOI] [PubMed] [Google Scholar]
- Piketty C., Derouin F., Rouveix B., Pocidalo J. J. In vivo assessment of antimicrobial agents against Toxoplasma gondii by quantification of parasites in the blood, lungs, and brain of infected mice. Antimicrob Agents Chemother. 1990 Aug;34(8):1467–1472. doi: 10.1128/aac.34.8.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]


