Abstract
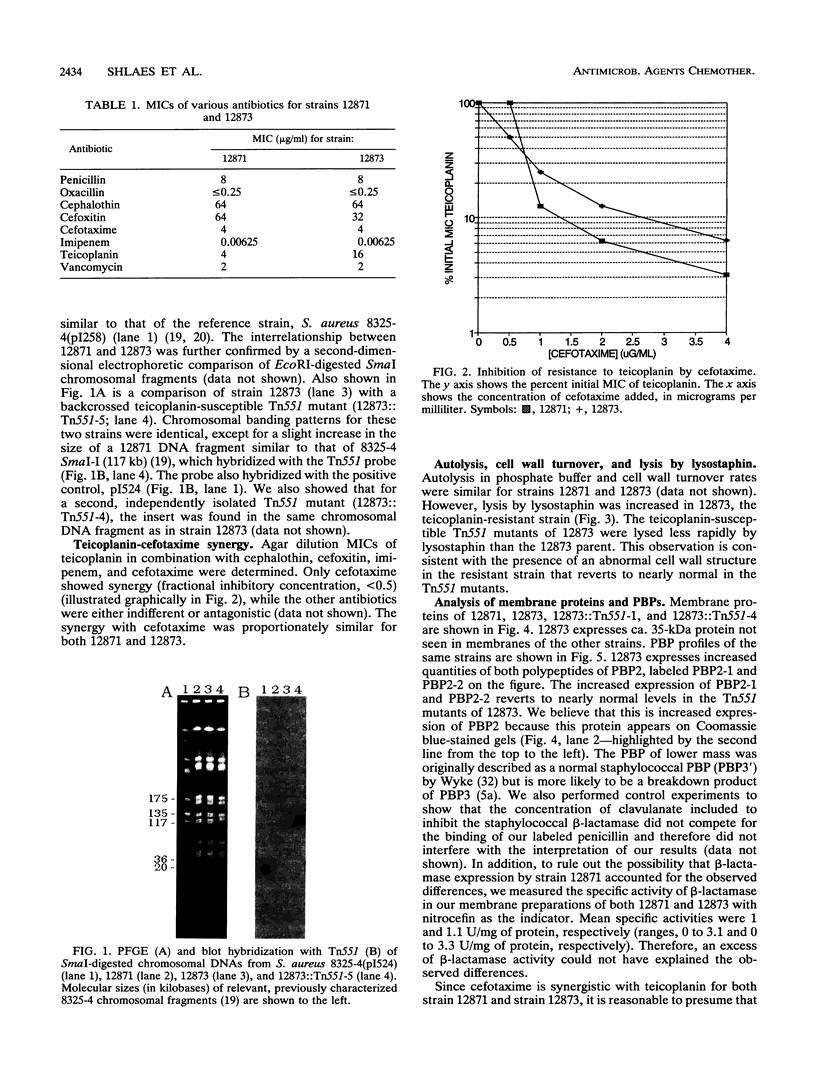
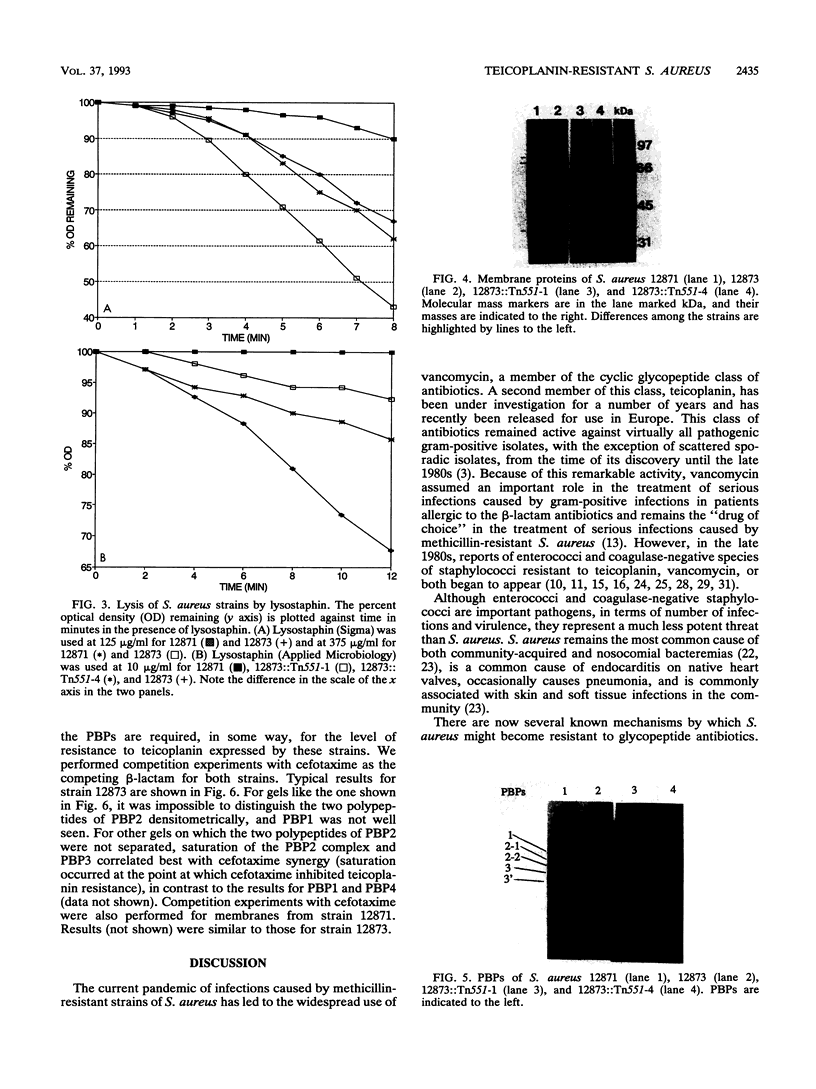
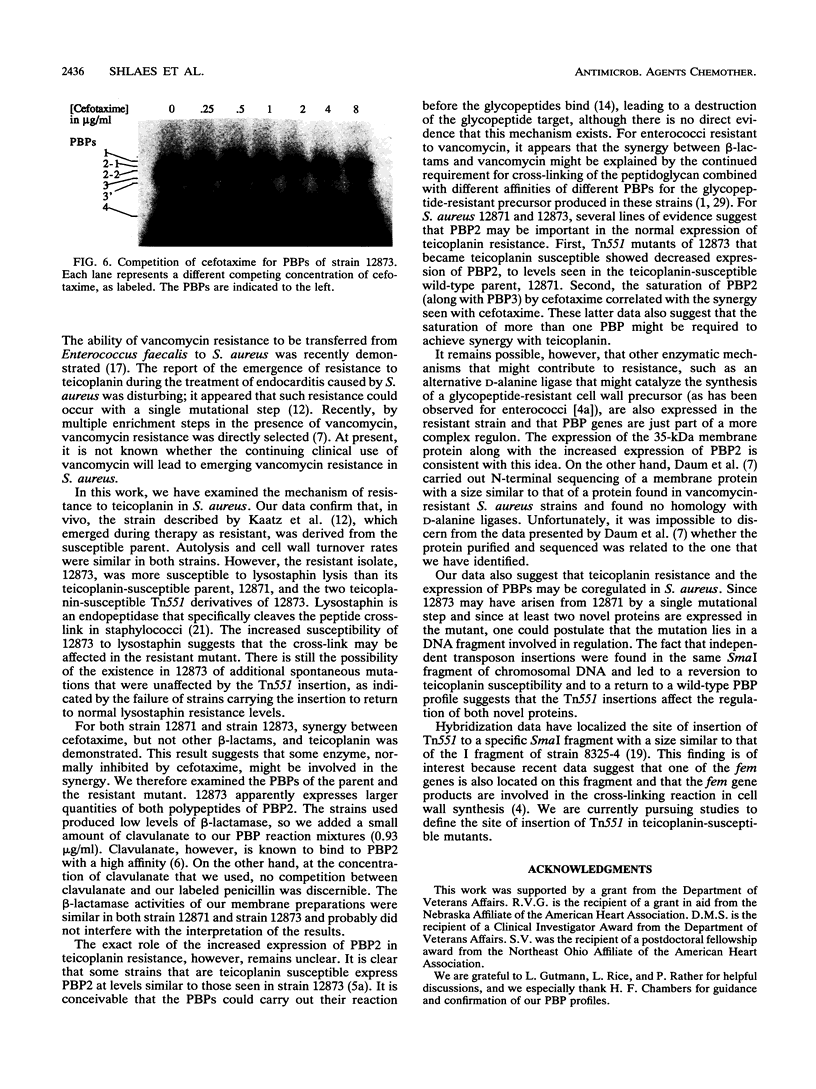
In the recent clinical trials of teicoplanin therapy of endocarditis caused by Staphylococcus aureus, at least one instance of the emergence of teicoplanin-resistant strains during therapy has been reported (G.W. Kaatz, S. M. Seo, N. J. Dorman, and S. A. Lerner, J. Infect. Dis 162:103-108, 1990). We have confirmed, using conventional electrophoresis of EcoRI-digested chromosomal DNA and pulsed-field gel electrophoresis of SmaI-digested chromosomal DNA, that the resistant strain (12873) (MIC, 16 micrograms/ml) is genetically very similar to the susceptible parent (12871) (MIC, 4 micrograms/ml). Kaatz et al. were able to select spontaneous teicoplanin-resistant mutants (10(-9)), suggesting that a single gene might be involved. We have shown that the mutation is highly stable during growth in the absence of teicoplanin. Using Tn551, we have selected insertion mutants of 12873 that become teicoplanin susceptible. We have examined a number of aspects of cell wall physiology in strains 12871 and 12873 and the teicoplanin-susceptible Tn551 mutants of 12873. 12873 was more susceptible to lysostaphin lysis than 12871 and the susceptible Tn551 derivatives of 12873. Autolysis in phosphate buffer (pH 7.5) and cell wall turnover rates were similar in 12871 and 12873. An analysis of membrane proteins revealed the expression of a ca. 35-kDa protein and increased expression of both polypeptides of penicillin-binding protein (PBP) 2 (PBP2) in 12873 relative to 12871 and the Tn551 mutants of 12873. This increased expression was not related to PBP2', since both strains were susceptible to oxacillin in 2% NaCl (MIC, < or = 0.25 microgram/ml) and cellular DNA from neither strain hybridized with a specific mec gene probe. Two independent Tn551 inserts have been mapped to a ca. 117-kb SmaI fragment of the chromosome. These data suggest the possibility that the mutation resulting in resistance to teicoplanin involves the regulation of expression of both polypeptides of PBP2 and a 35-kDa membrane protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barna J. C., Williams D. H. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984;38:339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Gustafson J. E., Kayser F. H. Mapping and characterization of multiple chromosomal factors involved in methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Jul;36(7):1367–1373. doi: 10.1128/aac.36.7.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T. D., Dutka-Malen S., Arthur M., Courvalin P., Walsh C. T. Identification of vancomycin resistance protein VanA as a D-alanine:D-alanine ligase of altered substrate specificity. Biochemistry. 1991 Feb 26;30(8):2017–2021. doi: 10.1021/bi00222a002. [DOI] [PubMed] [Google Scholar]
- Chambers H. F. Coagulase-negative staphylococci resistant to beta-lactam antibiotics in vivo produce penicillin-binding protein 2a. Antimicrob Agents Chemother. 1987 Dec;31(12):1919–1924. doi: 10.1128/aac.31.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Miick C. Characterization of penicillin-binding protein 2 of Staphylococcus aureus: deacylation reaction and identification of two penicillin-binding peptides. Antimicrob Agents Chemother. 1992 Mar;36(3):656–661. doi: 10.1128/aac.36.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum R. S., Gupta S., Sabbagh R., Milewski W. M. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomycin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J Infect Dis. 1992 Nov;166(5):1066–1072. doi: 10.1093/infdis/166.5.1066. [DOI] [PubMed] [Google Scholar]
- Froggatt J. W., Johnston J. L., Galetto D. W., Archer G. L. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989 Apr;33(4):460–466. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Winters M. A. Rapid method for epidemiological evaluation of gram-positive cocci by field inversion gel electrophoresis. J Clin Microbiol. 1992 Mar;30(3):577–580. doi: 10.1128/jcm.30.3.577-580.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Uttley A. H., Woodford N., George R. C. Resistance to vancomycin and teicoplanin: an emerging clinical problem. Clin Microbiol Rev. 1990 Jul;3(3):280–291. doi: 10.1128/cmr.3.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Dorman N. J., Lerner S. A. Emergence of teicoplanin resistance during therapy of Staphylococcus aureus endocarditis. J Infect Dis. 1990 Jul;162(1):103–108. doi: 10.1093/infdis/162.1.103. [DOI] [PubMed] [Google Scholar]
- Karchmer A. W. Staphylococcal endocarditis. Laboratory and clinical basis for antibiotic therapy. Am J Med. 1985 Jun 28;78(6B):116–127. doi: 10.1016/0002-9343(85)90374-2. [DOI] [PubMed] [Google Scholar]
- Knox J. R., Pratt R. F. Different modes of vancomycin and D-alanyl-D-alanine peptidase binding to cell wall peptide and a possible role for the vancomycin resistance protein. Antimicrob Agents Chemother. 1990 Jul;34(7):1342–1347. doi: 10.1128/aac.34.7.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Patel A. H., Foster T. J., Pattee P. A. Physical and genetic mapping of the protein A gene in the chromosome of Staphylococcus aureus 8325-4. J Gen Microbiol. 1989 Jul;135(7):1799–1807. doi: 10.1099/00221287-135-7-1799. [DOI] [PubMed] [Google Scholar]
- Pattee P. A. Distribution of Tn551 insertion sites responsible for auxotrophy on the Staphylococcus aureus chromosome. J Bacteriol. 1981 Jan;145(1):479–488. doi: 10.1128/jb.145.1.479-488.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P. A., Gruss A. D., Novick R. P. Cloning, sequence, and expression of the lysostaphin gene from Staphylococcus simulans. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1127–1131. doi: 10.1073/pnas.84.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Culver D. H., Gaynes R. P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991 Sep 16;91(3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- Scheckler W. E., Scheibel W., Kresge D. Temporal trends in septicemia in a community hospital. Am J Med. 1991 Sep 16;91(3B):90S–94S. doi: 10.1016/0002-9343(91)90350-7. [DOI] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Vincent S., Minkler P., Bincziewski B., Etter L., Shlaes D. M. Vancomycin resistance in Enterococcus gallinarum. Antimicrob Agents Chemother. 1992 Jul;36(7):1392–1399. doi: 10.1128/aac.36.7.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D. A., Goering R. V. Tn4201, a beta-lactamase transposon in Staphylococcus aureus. Antimicrob Agents Chemother. 1988 Aug;32(8):1164–1169. doi: 10.1128/aac.32.8.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Billot-Klein D., van Heijenoort J., Collatz E., Gutmann L. Replacement of the essential penicillin-binding protein 5 by high-molecular mass PBPs may explain vancomycin-beta-lactam synergy in low-level vancomycin-resistant Enterococcus faecium D366. FEMS Microbiol Lett. 1992 Feb 1;70(1):79–84. doi: 10.1016/0378-1097(92)90566-7. [DOI] [PubMed] [Google Scholar]






