Abstract
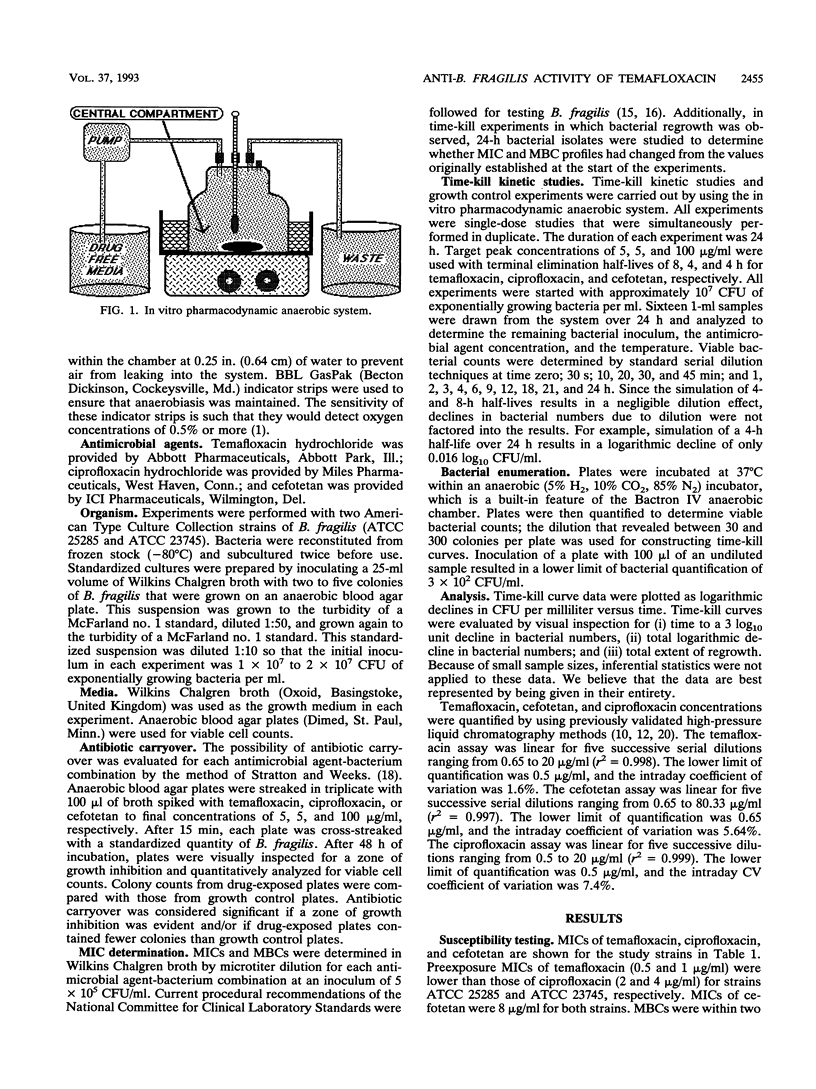
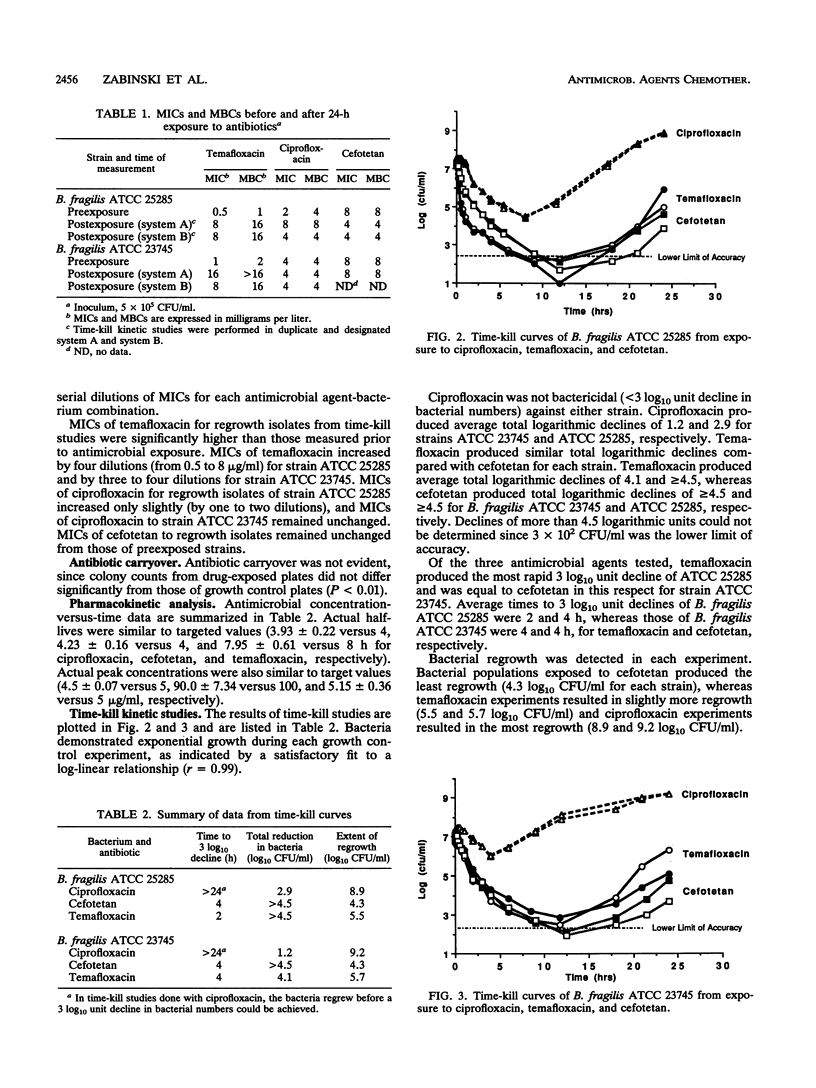
An in vitro pharmacodynamic system has been successfully adapted to simulate in vivo antimicrobial pharmacokinetics under anaerobic conditions. This system was used to perform time-kill kinetic studies which were designed to compare the activity of temafloxacin to ciprofloxacin and cefotetan against two strains of Bacteroides fragilis (ATCC 25285 and ATCC 23745). All experiments were performed as single-dose, 24-h, duplicate runs. Starting bacterial inocula of 10(7) CFU/ml were exposed to starting antimicrobial concentrations of 5 micrograms of temafloxacin per ml, 5 micrograms of ciprofloxacin per ml, and 100 micrograms of cefotetan per ml. Terminal half-lives of 8, 4, and 4 h were simulated for each antimicrobial agent. Temafloxacin was rapidly bactericidal against B. fragilis. Ciprofloxacin was not bactericidal (< 3 log10 unit decline in bacterial numbers) to either strain of B. fragilis. Cefotetan was bactericidal (> or = 3 log10 unit decline in bacterial numbers) to each strain but killed at a slower rate than temafloxacin. Times to 3 log10 unit declines of strain ATCC 25285 were 2, 4, and > 24 h, whereas those of strain ATCC 23745 were 4, 4, and > 24 h for temafloxacin, cefotetan, and ciprofloxacin, respectively. Total logarithmic declines of strain ATCC 25285 were > 4.5, > 4.5, and 2.9 log10 CFU/ml, whereas those of strain ATCC 23745 were 4.1, > 4.5, and 1.2 log10 CFU/ml for each drug, respectively. These and other studies demonstrated that temafloxacin showed potential as an agent that could have been further developed for use in the treatment of anaerobic infections. However, the drug was removed from the market by its manufacturer because of toxicity issues. Although the release of newer fluoroquinolones that possess significant activity against anaerobic bacteria does not appear imminent, the time-kill studies performed in this study demonstrate that further research is warranted in the development of fluoroquinolones which possess significant antianaerobic activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser J., Stone B. B., Groner M. C., Zinner S. H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987 Jul;31(7):1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L., Johnson D. E., Rosen M., Standiford H. C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993 Mar;37(3):483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M. N., Blaser J., Gilbert D., Mayer K. H., Zinner S. H. Combination therapy with ciprofloxacin plus azlocillin against Pseudomonas aeruginosa: effect of simultaneous versus staggered administration in an in vitro model of infection. J Infect Dis. 1991 Sep;164(3):499–506. doi: 10.1093/infdis/164.3.499. [DOI] [PubMed] [Google Scholar]
- Garrison M. W., Vance-Bryan K., Larson T. A., Toscano J. P., Rotschafer J. C. Assessment of effects of protein binding on daptomycin and vancomycin killing of Staphylococcus aureus by using an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1990 Oct;34(10):1925–1931. doi: 10.1128/aac.34.10.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. U., Vastola A. P., Brandel J., Craig W. A. Selection of aminoglycoside-resistant variants of Pseudomonas aeruginosa in an in vivo model. J Infect Dis. 1982 Nov;146(5):691–697. doi: 10.1093/infdis/146.5.691. [DOI] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M. Comparative activity of ciprofloxacin, ofloxacin, sparfloxacin, temafloxacin, CI-960, CI-990, and WIN 57273 against anaerobic bacteria. Antimicrob Agents Chemother. 1992 May;36(5):1158–1162. doi: 10.1128/aac.36.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath B. J., Bailey E. M., Lamp K. C., Rybak M. J. Pharmacodynamics of once-daily amikacin in various combinations with cefepime, aztreonam, and ceftazidime against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob Agents Chemother. 1992 Dec;36(12):2741–2746. doi: 10.1128/aac.36.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye K., Shi Y. G., Andrews J. M., Ashby J. P., Wise R. The in-vitro activity, pharmacokinetics and tissue penetration of temafloxacin. J Antimicrob Chemother. 1989 Sep;24(3):415–424. doi: 10.1093/jac/24.3.415. [DOI] [PubMed] [Google Scholar]
- Sato K., Hoshino K., Tanaka M., Hayakawa I., Osada Y. Antimicrobial activity of DU-6859, a new potent fluoroquinolone, against clinical isolates. Antimicrob Agents Chemother. 1992 Jul;36(7):1491–1498. doi: 10.1128/aac.36.7.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Weeks L. S. Effect of human serum on the bactericidal activity of daptomycin and vancomycin against staphylococcal and enterococcal isolates as determined by time-kill kinetic studies. Diagn Microbiol Infect Dis. 1990 May-Jun;13(3):245–252. doi: 10.1016/0732-8893(90)90067-6. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]


