Abstract
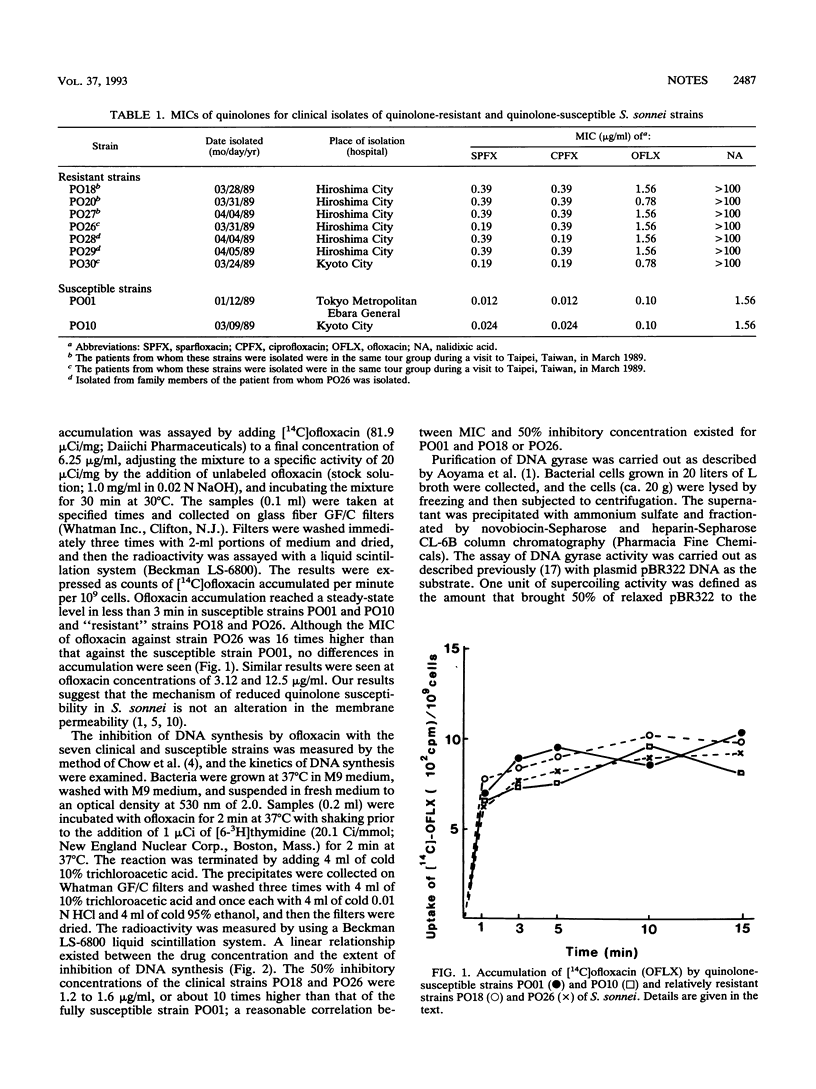
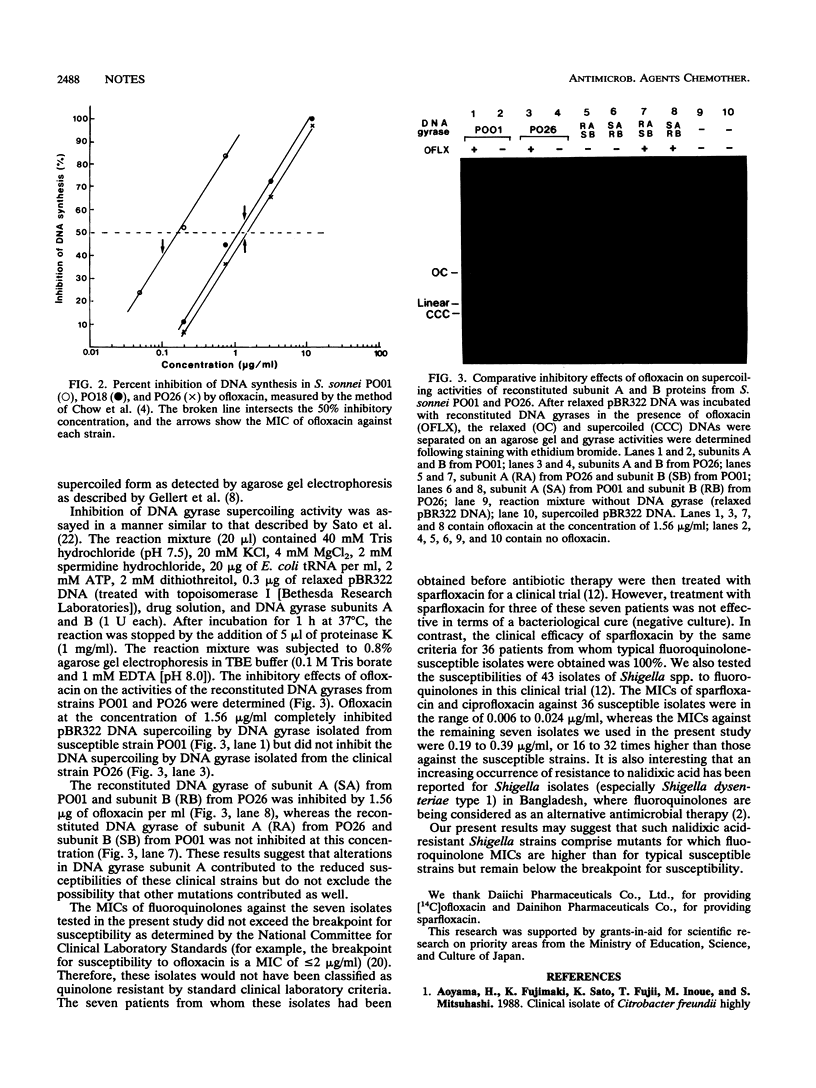
Seven clinical isolates of Shigella sonnei with reduced susceptibilities to fluoroquinolones (sparfloxacin, ciprofloxacin, and ofloxacin) were obtained. The MICs of fluoroquinolones against these S. sonnei strains were 16 to 32 times higher than those obtained against typical strains that are highly susceptible to these agents. The kinetics of [14C]ofloxacin accumulation in these clinical strains were not different from those in the fully susceptible strains. However, DNA synthesis was much less inhibited by ofloxacin in the strains with reduced susceptibility. Analysis of the in vitro activity of the partially purified DNA gyrase from these isolates showed that the decreased quinolone susceptibility of the S. sonnei strains was likely due to mutation of the DNA gyrase subunit A gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama H., Fujimaki K., Sato K., Fujii T., Inoue M., Hirai K., Mitsuhashi S. Clinical isolate of Citrobacter freundii highly resistant to new quinolones. Antimicrob Agents Chemother. 1988 Jun;32(6):922–924. doi: 10.1128/aac.32.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennish M. L., Salam M. A., Hossain M. A., Myaux J., Khan E. H., Chakraborty J., Henry F., Ronsmans C. Antimicrobial resistance of Shigella isolates in Bangladesh, 1983-1990: increasing frequency of strains multiply resistant to ampicillin, trimethoprim-sulfamethoxazole, and nalidixic acid. Clin Infect Dis. 1992 May;14(5):1055–1060. doi: 10.1093/clinids/14.5.1055. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Ciprofloxacin, a quinolone carboxylic acid compound active against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984 Mar;25(3):319–326. doi: 10.1128/aac.25.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R. T., Dougherty T. J., Fraimow H. S., Bellin E. Y., Miller M. H. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob Agents Chemother. 1988 Aug;32(8):1113–1118. doi: 10.1128/aac.32.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Hooper D. C., Wolfson J. S., Levy S. B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989 Aug;33(8):1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook S. M., Selkon J. B., McLardy Smith P. D. Clinical resistance to long-term oral ciprofloxacin. Lancet. 1985 Jun 1;1(8440):1275–1275. doi: 10.1016/s0140-6736(85)92343-8. [DOI] [PubMed] [Google Scholar]
- Fujimaki K., Noumi T., Saikawa I., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activities of T-3262, a new fluoroquinolone. Antimicrob Agents Chemother. 1988 Jun;32(6):827–833. doi: 10.1128/aac.32.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985 Nov;28(5):716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresken M., Wiedemann B. Development of resistance to nalidixic acid and the fluoroquinolones after the introduction of norfloxacin and ofloxacin. Antimicrob Agents Chemother. 1988 Aug;32(8):1285–1288. doi: 10.1128/aac.32.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T., Neu H. C. In-vitro activity of ofloxacin, a quinolone carboxylic acid compared to other quinolones and other antimicrobial agents. J Antimicrob Chemother. 1985 Nov;16(5):563–574. doi: 10.1093/jac/16.5.563. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Scurlock T. R. DNA gyrase (Topoisomerase II) from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1983 Jan 27;110(2):694–700. doi: 10.1016/0006-291x(83)91205-6. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniot-Ville N., Guibert J., Moreau N., Acar J. F., Collatz E., Gutmann L. Mechanisms of quinolone resistance in a clinical isolate of Escherichia coli highly resistant to fluoroquinolones but susceptible to nalidixic acid. Antimicrob Agents Chemother. 1991 Mar;35(3):519–523. doi: 10.1128/aac.35.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya R., Rownd R. Transduction of R factors by a Proteus mirabilis bacteriophage. J Bacteriol. 1971 Jun;106(3):773–783. doi: 10.1128/jb.106.3.773-783.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E. Effect of new quinolones on the human gastrointestinal microflora. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S193–S196. doi: 10.1093/clinids/10.supplement_1.s193. [DOI] [PubMed] [Google Scholar]
- Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segreti J., Nelson J. A., Goodman L. J., Kaplan R. L., Trenholme G. M. In vitro activities of lomefloxacin and temafloxacin against pathogens causing diarrhea. Antimicrob Agents Chemother. 1989 Aug;33(8):1385–1387. doi: 10.1128/aac.33.8.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. L., Kohlbrenner W. E., Weigl D., Baranowski J. Mechanism of quinolone inhibition of DNA gyrase. Appearance of unique norfloxacin binding sites in enzyme-DNA complexes. J Biol Chem. 1989 Feb 15;264(5):2973–2978. [PubMed] [Google Scholar]
- Sugino A., Cozzarelli N. R. The intrinsic ATPase of DNA gyrase. J Biol Chem. 1980 Jul 10;255(13):6299–6306. [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Yamanaka L. M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991 Aug;35(8):1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]



