Abstract
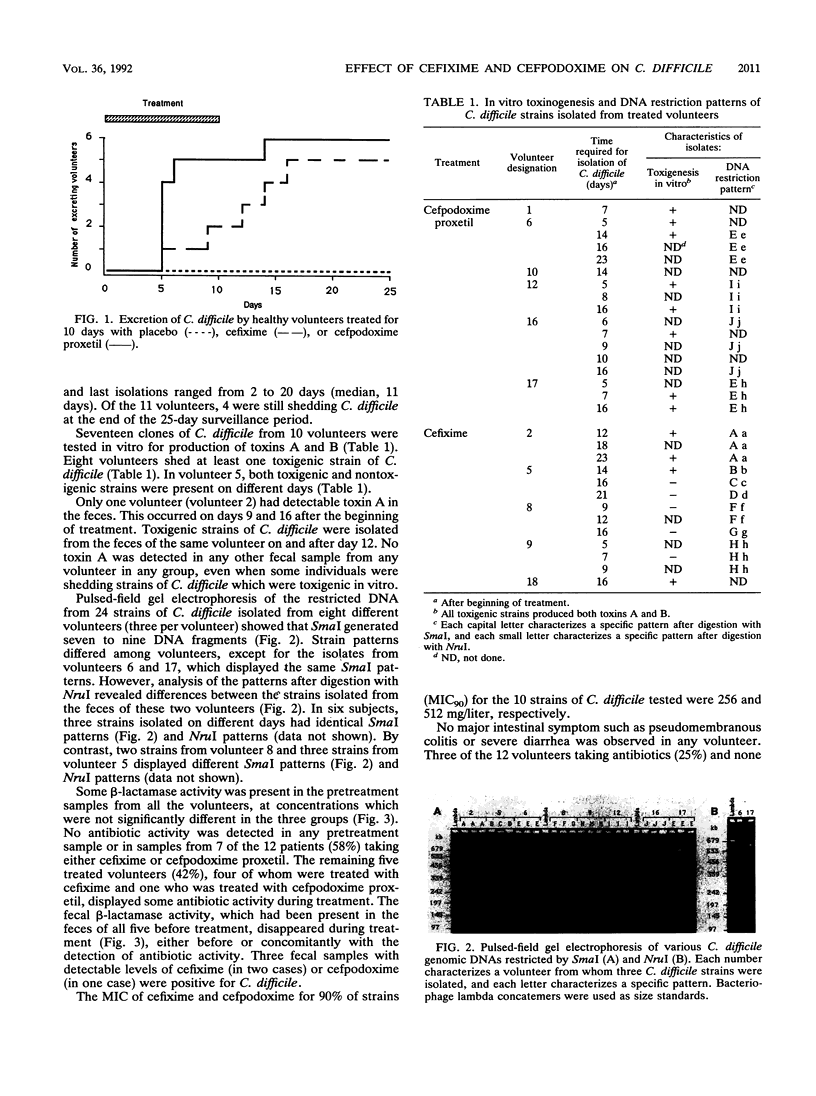
Three groups of six healthy adult volunteers were randomly assigned to a treatment with 400 mg of oral cefpodoxime proxetil, oral cefixime, or placebo per day for 10 days. Informed consent was obtained from all volunteers. Clostridium difficile was not detected in the feces of any subject before treatment or at any time in the subjects in the placebo group. C. difficile was, however, detected in all subjects treated with cefpodoxime proxetil and in five of six treated with cefixime. Genomic DNA restriction patterns showed that the strains of C. difficile differed from one volunteer to another. Two subjects both shed different strains at different times during the 25-day surveillance period. All isolates were resistant to cefixime and cefpodoxime (MIC for 90% of strains, 256 and 512 mg/liter, respectively). Antibiotic activity was found in the feces of one volunteer treated with cefpodoxime proxetil and of four volunteers treated with cefixime. It was inversely correlated with the presence of fecal beta-lactamase activity. Intestinal side effects were limited to modifications of stool consistency, which occurred in only 3 of the 12 treated volunteers and did not lead to cessation of treatment. These modifications were significantly associated with the presence of fecal antibiotic activity (P less than 0.05) but not with the shedding of toxigenic or nontoxigenic strains of C. difficile or with the presence of toxin A in feces, which was detected only in one perfectly healthy treated volunteer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y., BOULINGRE H. Modifications pratiques concernant le dosage des antibiotiques en clinique. Rev Fr Etud Clin Biol. 1957 Jun;2(6):636–640. [PubMed] [Google Scholar]
- Canard B., Cole S. T. Genome organization of the anaerobic pathogen Clostridium perfringens. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6676–6680. doi: 10.1073/pnas.86.17.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen M. R., Bonnén H., Tvede M., Mortensen P. B. Colonic fermentation to short-chain fatty acids is decreased in antibiotic-associated diarrhea. Gastroenterology. 1991 Dec;101(6):1497–1504. doi: 10.1016/0016-5085(91)90384-w. [DOI] [PubMed] [Google Scholar]
- Corthier G., Dubos F., Raibaud P. Modulation of cytotoxin production by Clostridium difficile in the intestinal tracts of gnotobiotic mice inoculated with various human intestinal bacteria. Appl Environ Microbiol. 1985 Jan;49(1):250–252. doi: 10.1128/aem.49.1.250-252.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Girolami P. C., Hanff P. A., Eichelberger K., Longhi L., Teresa H., Pratt J., Cheng A., Letourneau J. M., Thorne G. M. Multicenter evaluation of a new enzyme immunoassay for detection of Clostridium difficile enterotoxin A. J Clin Microbiol. 1992 May;30(5):1085–1088. doi: 10.1128/jcm.30.5.1085-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M., Ingram-Drake L., Gee R., Reinhardt J., Edelstein M. A., MacDonald K., Wexler H. Bowel flora changes in humans receiving cefixime (CL 284,635) or cefaclor. Antimicrob Agents Chemother. 1987 Mar;31(3):443–446. doi: 10.1128/aac.31.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani A., Richard G. A., Johnson D., Bryant A. A double-blind, multicenter, comparative study of the safety and efficacy of cefixime versus amoxicillin in the treatment of acute urinary tract infections in adult patients. Am J Med. 1988 Sep 16;85(3A):17–23. doi: 10.1016/0002-9343(88)90459-7. [DOI] [PubMed] [Google Scholar]
- Kaatz G. W., Gitlin S. D., Schaberg D. R., Wilson K. H., Kauffman C. A., Seo S. M., Fekety R. Acquisition of Clostridium difficile from the hospital environment. Am J Epidemiol. 1988 Jun;127(6):1289–1294. doi: 10.1093/oxfordjournals.aje.a114921. [DOI] [PubMed] [Google Scholar]
- Kiani R., Johnson D., Nelson B. Comparative, multicenter studies of cefixime and amoxicillin in the treatment of respiratory tract infections. Am J Med. 1988 Sep 16;85(3A):6–13. doi: 10.1016/0002-9343(88)90457-3. [DOI] [PubMed] [Google Scholar]
- Knapp C. C., Sierra-Madero J., Washington J. A. Antibacterial activities of cefpodoxime, cefixime, and ceftriaxone. Antimicrob Agents Chemother. 1988 Dec;32(12):1896–1898. doi: 10.1128/aac.32.12.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh D. A., Walsh B., Leung A., Tait S., Peatey K., Hancock P. The effect of cefuroxime axetil on the faecal flora of healthy volunteers. J Antimicrob Chemother. 1990 Aug;26(2):261–268. doi: 10.1093/jac/26.2.261. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Sullivan N. M., Wilkins T. D. Enzyme-linked immunosorbent assay for Clostridium difficile toxin A. J Clin Microbiol. 1983 Jan;17(1):72–78. doi: 10.1128/jcm.17.1.72-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard F., Andremont A., Leclerq B., Labia R., Tancrède C. Use of beta-lactamase-producing anaerobes to prevent ceftriaxone from degrading intestinal resistance to colonization. J Infect Dis. 1989 Aug;160(2):274–280. doi: 10.1093/infdis/160.2.274. [DOI] [PubMed] [Google Scholar]
- McFarland L. V., Elmer G. W., Stamm W. E., Mulligan M. E. Correlation of immunoblot type, enterotoxin production, and cytotoxin production with clinical manifestations of Clostridium difficile infection in a cohort of hospitalized patients. Infect Immun. 1991 Jul;59(7):2456–2462. doi: 10.1128/iai.59.7.2456-2462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland L. V., Mulligan M. E., Kwok R. Y., Stamm W. E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989 Jan 26;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Movin G., Stålberg D. Impact of cefixime on the normal intestinal microflora. Scand J Infect Dis. 1988;20(5):547–552. doi: 10.3109/00365548809032504. [DOI] [PubMed] [Google Scholar]
- Periti P., Novelli A., Schildwachter G., Schmidt-Gayk H., Ryo Y., Zuck P. Efficacy and tolerance of cefpodoxime proxetil compared with co-amoxiclav in the treatment of exacerbations of chronic bronchitis. J Antimicrob Chemother. 1990 Dec;26 (Suppl E):63–69. doi: 10.1093/jac/26.suppl_e.63. [DOI] [PubMed] [Google Scholar]
- Prevost G., Jaulhac B., Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992 Apr;30(4):967–973. doi: 10.1128/jcm.30.4.967-973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. S., Edwards C. A., Austen C. J., Bruce C., Read N. W. Impaired colonic fermentation of carbohydrate after ampicillin. Gastroenterology. 1988 Apr;94(4):928–932. doi: 10.1016/0016-5085(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Intestinal beta-lactamase activity in ampicillin-induced, Clostridium difficile-associated ileocecitis. J Infect Dis. 1983 Feb;147(2):227–235. doi: 10.1093/infdis/147.2.227. [DOI] [PubMed] [Google Scholar]
- Safran C. Cefpodoxime proxetil: dosage, efficacy and tolerance in adults suffering from respiratory tract infections. J Antimicrob Chemother. 1990 Dec;26 (Suppl E):93–101. doi: 10.1093/jac/26.suppl_e.93. [DOI] [PubMed] [Google Scholar]
- Viscidi R., Willey S., Bartlett J. G. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981 Jul;81(1):5–9. [PubMed] [Google Scholar]
- Wilson K. H., Kennedy M. J., Fekety F. R. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol. 1982 Mar;15(3):443–446. doi: 10.1128/jcm.15.3.443-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. The pharmacokinetics of the oral cephalosporins--a review. J Antimicrob Chemother. 1990 Dec;26 (Suppl E):13–20. doi: 10.1093/jac/26.suppl_e.13. [DOI] [PubMed] [Google Scholar]



