Abstract
Systemin is a wound-signaling peptide that mediates defenses of tomato plants against herbivorous insects. Perception of systemin by the membrane-bound receptor SR160 results in activation of MAPKs, synthesis of jasmonic acid (JA), and expression of defense genes. To test the function of MAPKs in the response to systemin, we used virus-induced gene silencing (VIGS) in plants that overexpress the systemin precursor prosystemin (35S::prosys plants). These transgenic plants accumulate high levels of defense proteins and exhibit increased resistance to herbivorous insects. Cosilencing of the MAPKs MPK1 and MPK2 reduced MPK1/2 kinase activity, JA biosynthesis, and expression of JA-dependent defense genes. Application of methyl-JA restored the full defense response. These data show that MPK1 and MPK2 are essential components of the systemin signaling pathway and most likely function upstream of JA biosynthesis. MPK1 and MPK2 are 95% identical at the amino acid level. Specific VIGS of only MPK1 or MPK2 resulted in the same reduction of defense gene expression as cosilencing of MPK1 and MPK2, indicating that gene dosage effects may be important for MPK signaling. In addition, VIGS of the closely related MPK3 also reduced systemin-induced defense responses. The function of MPK1/2 and orthologs in pathogen-induced defenses is well established. Here we show that cosilencing of MPK1 and MPK2 compromised prosystemin-mediated resistance to Manduca sexta (Lepidoptera) herbivory, demonstrating that MPK1 and MPK2 are also required for successful defenses against herbivorous insects.
Keywords: jasmonic acid, plant–insect interactions, virus-induced gene silencing
Plants defend themselves against attacks by herbivorous insects via synthesis of toxic secondary metabolites and proteins such as proteinase inhibitors (PIs), polyphenol oxidase, and amino acid-catabolizing enzymes. These wound-response proteins prevent uptake of essential amino acids in insect intestines, thus causing growth and developmental defects (1–3). In tomato plants, the response to insect attacks and mechanical wounding is mediated by systemin, an 18-aa signaling peptide active at femtomolar concentrations (4). Systemin is derived from the precursor protein prosystemin (5). Prosystemin reduction-of-function plants exhibited low levels of defensive proteins and suffered severe defoliation by Manduca sexta, whereas larval growth was strongly increased compared with larvae feeding on WT plants (6). Overexpression of prosystemin caused the continuous synthesis and accumulation of defensive proteins. This correlated with increased tolerance to M. sexta larvae (1). Systemin is required for a successful systemic wound response (7, 8). However, the plant hormone jasmonic acid (JA), or a JA derivative, is the most likely long-distance wound signal (8–11). Perception of systemin by the membrane-bound receptor kinase SR160 initiates a signaling pathway that involves ion fluxes (3, 12, 13), MAPKs (12, 14, 15), calcium-dependent protein kinases (13, 16), ethylene (17, 18), reactive oxygen species (19, 20), and synthesis and action of JA via the octadecanoid pathway (8, 21–23). The earliest transcriptional response includes activation of JA-biosynthetic genes and prosystemin within 1 hr. These genes are collectively referred to as “early genes.” Transcript levels of effector proteins such as several groups of PIs, polyphenol oxidase, threonine deaminase, arginase, and leucine aminopeptidase start to increase later and reach maximal levels between 6 and 12 hr after systemin application or wounding (3, 7, 20, 24). They are referred to as “late genes.” Basal transcript levels of early genes are present in JA-insensitive coi1 null-mutant plants but increase in response to exogenous methyl-JA (MeJA) in a CORANATINE INSENSITIVE 1 (COI1)-dependent manner (24). Furthermore, the wound-induced expression of some early genes is partially JA-independent (25). In contrast to the early genes, late gene expression is completely JA-dependent (24).
It is not known where MAPKs function in the systemin- and wounding-induced signaling pathway. MAPKs are a part of a three-tiered phosphorelay cascade consisting of the MAPKs (MPKs), which are activated by MAPK kinases (MPKK or MKKs), which in turn are activated by MAPKK kinases (MAPKKKs). We had shown earlier that mechanical wounding with a hemostat and systemin application results in the activation of two MAPKs in tomato (Lycopersicon esculentum), LeMPK1 and LeMPK2, which belong to the A2 subgroup of plant MAPKs (12, 14, 15). Wounding also activated LeMPK3, which belongs to the A1 subgroup (12, 26). MPK1 and MPK2 are 95% identical at the amino acid level and coordinately activated by all stimuli we tested so far. MPK1/2 activation was not reduced in the def1 JA-biosynthesis mutant, indicating that these MAPKs function either upstream or in parallel to JA biosynthesis (15). MPK1 and -2 activity is regulated posttranslationally by phosphorylation. Transcript and protein levels were not altered in response to systemin and wounding. In contrast, LeMPK3 was transcriptionally up-regulated in response to wounding, whereas protein levels did not increase (12). Only a few plant MAPK substrates have been identified in in vivo studies, such as the 1-aminocyclopropane-1-carboxylate synthases 2 and 6, which are phosphorylated and activated by AtMPK6, an LeMPK1/2 ortholog (27).
LeMPK1, -2, and -3 were also activated by the host-specific elicitors AvrPto and AvrPtoB from Pseudomonas syringae (28) and infection by Xanthomonas campestris (26). Loss-of-function studies had shown they are required for defense against P. syringae and Mi-1-mediated resistance to aphids (29). In this study, we used virus-induced gene silencing (VIGS) and showed that these LeMPKs also function upstream of JA and are required for expression of a subset of wound-response genes and resistance to herbivorous insects.
Results
Cosilencing of LeMPK1 and LeMPK2 Reduces Systemin-Induced Late Gene Expression.
To functionally characterize LeMPKs, we used a loss-of-function approach with tobacco rattle virus (TRV)-mediated VIGS that had been optimized for tomato plants (30). Two Agrobacterium expression vectors (pTRV1 and pTRV2) carry the bipartite genome of TRV. Following known requirements for efficient gene silencing (31), we designed a cosilencing construct that targets MPK1 and -2 (pTRV-MPK1/2) but not MPK3. For controls, plants were coinfiltrated with pTRV1 and a pTRV2 vector that carries a partial sequence of GFP (pTRV-GFP) or an empty pTRV2 vector (pTRV) to exclude the possibility that some of the observed effects are due to general defense responses triggered by TRV or Agrobacterium.
To test whether MPK1 and MPK2 are essential components of the systemin signaling pathway, we infiltrated transgenic plants that overexpress prosystemin under the control of the cauliflower mosaic virus 35S promoter (35S::prosys plants) with the MPK1/2 cosilencing construct. 35S::prosys plants show a constitutive wound phenotype and slowly accumulate high levels of defensive proteins (32, 33). It is thought that the slow but continuous accumulation of PIs over time is due to the constant release of systemin (3), which leads to slightly higher steady-state JA levels than found in WT plants (23, 34). However, elevated JA levels could be detected only in young plants (23, 34). The transgenic plants had been used to isolate JA-insensitive and -biosynthetic mutants as suppressors of prosystemin-mediated responses, indicating that JA is essential for the prosystemin-mediated wound response (7, 32). We reasoned that mimicking MAPK mutants by using a VIGS approach in 35S::prosystemin (35S:prosys) plants will reveal whether MAPKs are essential components of the systemin signaling pathway. However, MAPK activity above background levels found in WT plants could not be detected in untreated 35S::prosys plants in in-gel kinase assaysand in immunocomplex kinase assays (Fig. 1 and data not shown). To verify that systemin specifically activates MPKs, we tested systemin-induced MPK activity in systemin-insensitive spr1 mutant plants (7). Although the oligosaccharide elicitor chitosan and stem excision resulted in increased MPK1, MPK2, and MPK3 activity in the leaves of WT and spr1 seedlings, systemin activated these MPKs only in WT plants [see supporting information (SI) Fig. 6].
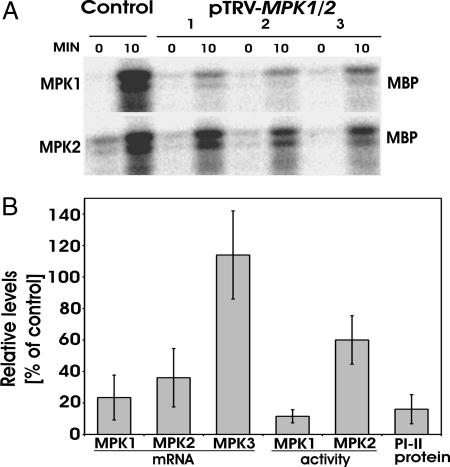
Fig. 1.
Cosilencing of MPK1 and MPK2 attenuates wound-induced MPK1/2 activity and PI-II synthesis. (A) 35S::prosys plants were infiltrated with either pTRV-MPK1/2 or -GFP (Control). Six weeks later, leaves were wounded and analyzed by immunocomplex kinase assays 0 and 10 min after wounding by using specific antibodies against MPK1 and MPK2. Signals represent phosphorylated myelin basic protein, an artificial MAPK substrate. Representative plants (1–3) are shown. (B) 35S::prosys plants from independent experiments were analyzed for MPK1 (n = 9), MPK2 (n = 9), and MPK3 (n = 5) mRNA levels by sqRT-PCR, for wound-induced MPK1 (n = 5), and MPK2 (n = 4) activity by immunocomplex kinase assays, and for PI-II protein levels by RIDA (n = 9). The levels in VIGS plants (mean ± SD) were expressed as percentages of the mean levels in control plants which were defined as 100%.
To show that VIGS of MPKs results in reduced MPK activity in transgenic plants, MAPK activity was induced by wounding. Compared with control plants, wound-induced MPK1 and MPK2 activity in pTRV-MPK1/2-infiltrated plants was reduced by 88% and 40%, respectively (Fig. 1). The combined reduction of MPK1/2 activity determined in in-gel kinase assays was 71 ± 9%. This correlated with reduction of MPK1 and MPK2 transcript levels by 77% and 64%, respectively (Fig. 1B). MPK3 transcript levels (Fig. 1B) and activity (not shown) were not significantly reduced in MPK1/2-cosilenced plants, demonstrating that the pTRV-MPK1/2 construct specifically targets MPK1 and MPK2. Not all plants exhibited strong silencing, and only plants with a reduction of MPK1/2 transcript levels by >50% were analyzed in further experiments.
To test whether silencing of MPK1/2 affects prosystemin-induced defense protein accumulation in untreated plants, we measured protein levels of PI-II, a marker for late gene expression in the tomato wound response. PI-II levels in control plants were high but were reduced by 84% in pTRV-MPK1/2-infiltrated plants (Fig. 1B). This demonstrates that MPK1 and MPK2 are essential components of the systemin signaling pathway. Because MAPKs are known to function only via their phosphotransfer activity, it is likely they are active in the 35S:prosys plants. However, this activity cannot be detected as significant in standard kinase assays.
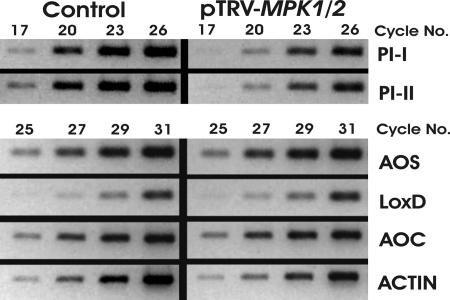
We also tested transcript levels of additional wound response marker genes in pTRV-MPK1/2-infiltrated 35S:prosys plants that showed reduced MPK1/2 transcript levels. PI-I is a late gene and belongs to a different gene family than PI-II. PI-I and PI-II transcript levels were strongly reduced in MPK1/2-cosilenced plants. Transcript levels of the early genes LIPOXYGENASE D (LoxD), ALLENE OXIDE SYNTHASE2 (AOS), and ALLENE OXIDE CYCLASE (AOC) are up-regulated by systemin (7, 23), but they were not significantly altered in MPK1/2-cosilenced plants (Fig. 2). In addition, LoxD mRNA increased 1 hr after wounding in MPK1/2-cosilenced plants to the same levels as in controls (data not shown). These data show that MPK1 and MPK2 regulate the expression of late JA-dependent genes.
Fig. 2.
Cosilencing of MPK1 and MPK2 attenuates expression of late systemin-induced wound response genes. 35S::prosys plants were infiltrated with pTRV-MPK1/2 and -GFP (Control). Five weeks later, transcript levels of Actin (internal control), the late genes PI-I and PI-II and the early genes AOS2, LoxD, and AOC were assessed by sqRT-PCR in leaf tissue. Ethidium bromide-stained agarose gels containing RT-PCR products are shown (colors inverted). The experiments are representative of 10 plants from three independent experiments for the late genes and of six plants from three independent experiments for the early genes.
Cosilencing of LeMPK1 and LeMPK2 Reduces Systemin-Induced JA Biosynthesis.
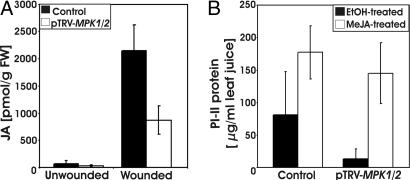
Because late gene expression is completely JA-dependent (24, 35), we hypothesized that LeMPKs would function upstream of JA synthesis. 35S::prosys plants were infiltrated with pTRV-MPK1/2, and 4 weeks later, plants were wounded, and JA levels were determined 1 hr after wounding. Silencing was confirmed by semiquantitative RT-PCR (sqRT-PCR). Unwounded 35S::prosys plants exhibited low background levels of JA. Because JA levels in unwounded control plants were highly variable (108 ± 96 pmol/g), we were unable to detect significant differences between control and cosilenced plants (35 ± 20 pmol/g). However, JA levels increased strongly after wounding in control plants, whereas wound-induced JA levels in cosilenced plants were 60% lower (Fig. 3A). Consistent with a role of MAPKs upstream of JA biosynthesis, we found that supplementation of MeJA to MPK1/2-cosilenced plants restored PI-II synthesis. PI-II levels in control plants were elevated because of the presence of the Prosystemin transgene and further increased in response to MeJA. MPK1/2-cosilenced plants showed low PI-II levels similar to those shown in Fig. 1B, but the levels increased to similar levels as in control plants in response to MeJA treatment (Fig. 3B). These data indicate that MPK1 and MPK2 function upstream of JA synthesis.
Fig. 3.
Cosilencing of MPK1 and MPK2 attenuates JA biosynthesis. (A) 35S::prosys plants were infiltrated with pTRV-MPK1/2 or -GFP (Control). Four weeks later, leaves were wounded, and JA levels were measured in unwounded and wounded leaves 1 hr later. The bars represent the mean ± SD in 10 plants from three independent experiments. (B) 35S::prosys plants were infiltrated with pTRV-MPK1/2 or -GFP (Control). Four weeks later, plants were exposed to MeJA vapor (open bars) or to the solvent ethanol (filled bars) in a closed environment for 12 hr. Twenty-four hours after the start of the experiment, PI-II protein levels in leaves were measured by RIDA. The bars represent mean ± SD (n ≥ 18; three independent experiments).
Silencing of LeMPK1, LeMPK2, and LeMPK3 Alone Has Similar Effects as Cosilencing of LeMPK1 and LeMPK2.
VIGS constructs were generated that specifically target either MPK1, MPK2, or MPK3 based on 3′-UTR sequences. The UTRs do not show significant sequence homology to each other. Four weeks after infiltration of 35S::prosys plants with either pTRV-MPK1 or pTRV-MPK2, transcript levels of the targeted MPK, but not of the respective other MPK, were reduced by ≈60% (Fig. 4 A–D). Reduced MPK1 and MPK2 transcript levels both correlated with an 86% and 72% reduction of systemin-induced PI-II synthesis, respectively (Fig. 4 B and D). PI-I and PI-II transcript levels were also reduced in both MPK1- and MPK2-silenced plants (Fig. 4 A and C). This shows that the presence of both MPK1 and MPK2 is required for late gene expression in response to systemin. Transcript levels for early genes were not significantly reduced but were more variable as compared with MPK1/2-cosilenced plants (data not shown).
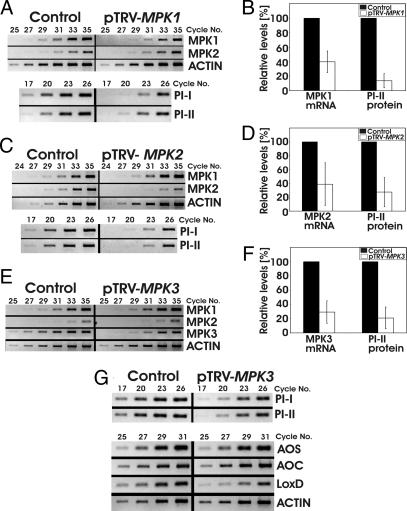
Fig. 4.
VIGS of individual MPKs attenuates systemin-induced late gene expression. 35S::prosys plants were infiltrated with pTRV-MPK1 (A and B), -MPK2 (C and D), -MPK3 (E–G), or -GFP (Control, A–G). Four weeks later, MPK, PI-I, and PI-II transcript levels and PI-II protein levels were determined in leaf tissue by sqRT-PCR and RIDAs, respectively. (A, C, and E) MPK, PI, and Actin transcript levels. Ethidium bromide-stained agarose gels of PCR products are shown (colors inverted). (B, D, and F) MPK transcript levels and PI-II protein levels. The levels in VIGS plants (mean ± SD) (open bars) were expressed as percentages of the mean levels in control plants (filled bars), which were defined as 100% (n ≥ 5; ≥ 2 independent experiments; mean PI-II in controls of B, D, and F: 78 ± 15, 61 ± 16, and 75 ± 14 μg/ml leaf juice, respectively). (G) Ethidium bromide-stained agarose gels of PCR products corresponding to the early genes AOS, AOC, and LoxD, and to the late genes PI-I and PI-II in pTRV-MPK3- and pTRV-GFP-infiltrated control plants (n = 8; four independent experiments).
In tobacco, the LeMPK3 ortholog WIPK had been shown to regulate wound-induced gene expression and JA synthesis (36, 37). VIGS of MPK3 reduced transcript levels of MPK3 by 71% and did not alter transcript levels of MPK1 and MPK2 (Fig. 4 E and F). This correlated with a reduction in systemin-induced PI-II synthesis by 79% (Fig. 4F). Transcript levels of the late genes PI-I and PI-II were also reduced in pTRV-MPK3-infiltrated plants, whereas transcript levels of the early genes AOS and AOC were not significantly altered (Fig. 4G). In addition, silencing of MPK3 resulted in significant reductions in LoxD transcript levels (Fig. 4G), unlike silencing of MPK1 and MPK2 and cosilencing of MPK1/2. Taken together, VIGS of each of the three LeMPKs revealed they all participate in regulating the expression of late wound-response genes.
Cosilencing of LeMPK1 and LeMPK2 Reduces Systemin-Mediated Resistance to M. sexta Larvae.
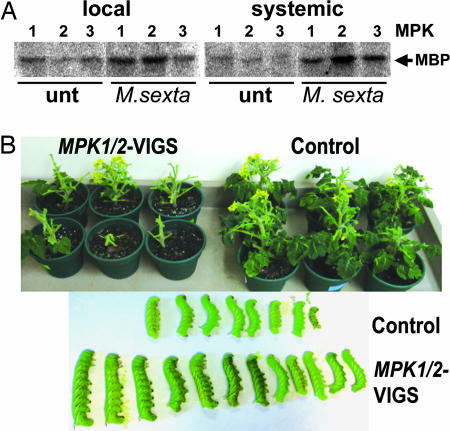
We have shown earlier that M. sexta larvae systemically induce 48-kDa MAPK activity in tomato seedlings (15). Using immunocomplex kinase assays with specific antibodies against MPK1, -2, and -3 (14), we show here that feeding M. sexta larvae activated MPK1 and MPK2 in the wounded leaf of two-leaf-stage WT plants. In the unwounded systemic leaf, all three MPKs were activated (Fig. 5A). To test whether cosilencing of MPK1/2 would affect the performance of herbivorous insects, we exposed 35S:prosys plants to M. sexta larvae 4 weeks after infiltration with pTRV-MPK1/2. Larvae were placed onto the plants 3–5 days after hatching and allowed to feed for 11 days. Most cosilenced plants were completely defoliated, whereas control plants maintained more than half of their foliage compared with unattacked control plants. The weight of the M. sexta larvae on the MPK1/2-cosilenced plants was 2.3 ± 0.2-fold higher than the weight of larvae feeding on the control plants (Fig. 5B). Silencing of the MPK1/2-cosilenced plants was confirmed by sqRT-PCR (data not shown).
Fig. 5.
M. sexta herbivory activates MPKs, and cosilencing of MPK1 and MPK2 reduces systemin-mediated resistance to M. sexta larvae. (A) M. sexta larvae were allowed to consume one-half of the terminal leaflet of the lower leaf of two-leaf stage WT tomato seedlings. Ten minutes after onset of feeding (M. sexta), the wounded (local) and unwounded (systemic) leaf and leaves of unwounded control plants (unt) were assayed for MPK1, MPK2, and MPK3 activity by an immunocomplex kinase assay. Phosphorylated myelin basic protein is shown. The experiment is representative of three similar experiments. (B) 35S::prosys plants were infiltrated with pTRV-MPK1/2 and -GFP (Control). Four weeks later, each plant was exposed to one M. sexta larva for 11 days. Leaf damage (Upper; 12 representative plants are shown) and larval size (Lower) were documented photographically. An additional experiment generated similar results (not shown).
Discussion
We investigated the role of tomato MAPKs in the systemin-mediated wound response in tomato plants. Cosilencing of MPK1 and MPK2 in WT plants reduced wound-induced 48-kDa MAPK activity and resulted in reduced PI-II synthesis as compared with control plants (data not shown). Wounding not only leads to the release of systemin but also generates additional MAPK-activating signals, such as rapidly propagated mechanical signals (15) or reactive oxygen species and oligosaccharide elicitors released at the site of wounding (3, 20). To specifically investigate the role of MAPKs in the response to systemin, we used transgenic 35S::prosys plants that constitutively produce and accumulate defense proteins without external treatments (32, 33). In these plants, we showed that silencing of MPK1, MPK2, and MPK3 attenuated prosystemin-mediated defense protein accumulation, demonstrating that these MPKs are essential components of the systemin-induced signaling pathway. The role that MPK3 plays in the wound response seems to be somewhat different from MPK1 and MPK2. Unlike VIGS of MPK1/2, VIGS of MPK3 lowered the transcript levels of LoxD in 35S::prosys plants, whereas transcripts of the other early genes AOS and AOC were not affected (Fig. 4G). In addition, we found increased MPK3 activity in response to M. sexta attack mainly in the systemic leaf, whereas MPK1 and -2 activity increased both in the wounded and the systemic leaf (Fig. 5A). Similarly, both different and overlapping roles in the response of Nicotiana tabacum and Nicotiana attenuata to wounding or M. sexta oral secretions were reported for SIPK and WIPK, the tobacco orthologs of tomato MPK1 and MPK3 (38, 39).
JA is an essential signaling component in the systemin and wound-signaling pathways (8). The reduced expression of the strictly JA-dependent late genes in MPK1-, MPK2-, MPK1/2-, and MPK3-silenced 35S::prosys plants (Figs. 1, 2, and 4) indicates these MAPKs could function either upstream or downstream of systemin-induced JA biosynthesis. Restoration of late gene expression by application of MeJA to MPK1/2-cosilenced unwounded 35S::prosys plants and reduced wound-induced JA synthesis in MPK1/2-cosilenced 35S::prosys plants demonstrate that these MAPKs function upstream of JA biosynthesis. Consequently, MPK1 and MPK2 (and possibly MPK3) represent a link between the cytosolic part of the systemin signaling pathway and JA synthesis, which is initiated in the chloroplasts. Substrates of the tomato MPKs are not known so far. Because transcript levels of JA-biosynthetic enzymes were not altered in MPK1/2-silenced plants, it is conceivable that the MPK substrate(s) regulate the activity of the JA-biosynthetic enzymes directly or indirectly, perhaps via substrate availability.
JA is known to interact with ethylene to activate wound-response genes (18). Recently, it was demonstrated that the LeMPK1/2 ortholog in Arabidopsis, AtMPK6, regulates ethylene synthesis through phosphorylation and activation of the cytosolic enzyme 1-aminocyclopropane-1-carboxylic acid synthase (ACS2/6) in vivo (27). Ethylene is known to be generated in response to systemin (17, 18), and it is possible that ACS is also the physiological substrate of MPK1/2 in the systemin signaling pathway. Consistent with this scenario is our observation that ethylene synthesis in wounded leaves of MPK1/2-silenced 35S::prosys plants is reduced by ≈30% (see SI Fig. 7). This is comparable to the reduction of ethylene synthesis in flagellin-treated Arabidopsis mpk6 null mutants (27), wounded SIPK-silenced tobacco (38), and N. attenuata treated with M. sexta oral secretions (39). These data do not exclude the possibility that MPK1/2 might recruit additional cytosolic or nuclear substrates.
MPK1 and MPK2 belong to the A2 subgroup of plant MAPKs and are 95% identical at the amino acid level (14). Both are activated by the same upstream MAPKKs, LeMKK2 and LeMKK4 (28). In addition, they are coordinately activated by wounding, systemin, oligosaccharide elicitors, UV-B radiation, and the fungal toxin fusicoccin (12, 14). These data suggested that the two MPKs are functionally redundant. Therefore, we did not expect to find a pronounced effect in plants silenced for only one of the two paralogs. In contrast, silencing of either MPK1 or MPK2 resulted in a strong reduction of PI-I and PI-II transcript accumulation and PI-II protein synthesis (Fig. 4), demonstrating that the presence of one of the two MPKs is not sufficient to induce a strong wound response. The tobacco genome also contains two highly homologous MAPKs of the A2 subgroup, SIPK and Ntf4. Recently, it was shown that their functions are largely redundant (40). The authors also concluded that some of the published loss-of-function studies that targeted SIPK (41, 42) most likely also silenced Ntf4, and that systematic loss-of-function studies of each of the two MAPK paralogs are lacking. They further suggested that the presence of two highly homologous MAPKs in certain solanaceous plants may be adaptive, either because the two MAPKs do have some different yet-unknown functions or because of a gene-dosage effect (40). Our results are consistent with the latter scenario. We speculate that the sum of MPK1 and MPK2 molecules per cell has to exceed a critical copy number to effectively signal gene expression, and that VIGS of only one of the two MPKs lowered this MPK1/2 copy number below a critical threshold. An investigation of the involvement of SIPK in the tobacco response to ozone showed that silencing of SIPK by RNAi did not affect Ntf4 transcript levels. Similar to our results, the SIPK-RNAi plants suffered more ozone-induced oxidative damage, indicating that Ntf4 alone is not sufficient to confer ozone resistance (43). MPK3-silencing also attenuated late gene expression. But the presence of MPK3 in MPK1/2-silenced plants did not prevent the reduction of defense protein accumulation in these plants (Fig. 1B). This indicates that MPK3 has a different mechanism of action than MPK1 and MPK2, e.g., activation of a different substrate. In tobacco, regulation of WIPK gene expression or activity by SIPK had been discussed (43, 44). We did not find evidence for such a scenario in untreated 35S::prosys plants. However, it cannot be excluded that MPK3 levels are altered in a MPK1/2-dependent manner in response to treatments.
LeMPK1/2/3 had been shown to function in host-specific AvrPto-dependent resistance to the bacterial pathogen P. syringae (28, 45) and in Mi-1-mediated resistance to aphids (29). Our reduction-of-function study demonstrates that LeMPK1 and LeMPK2 are required for systemin-mediated resistance to M. sexta larvae. Antisense expression of prosystemin in transgenic tomato plants resulted in reduced defense protein accumulation and thus loss of resistance to M. sexta (6), whereas 35S::prosys plants exhibited high constitutive levels of defense proteins and increased resistance to M. sexta (1) and other insects (46). MPK1 and MPK2 play an essential role for systemin-induced defense protein synthesis, and the presence of MPK3 cannot compensate for the loss of MPK1 and MPK2 (Fig. 4). Consistent with these data, VIGS of MPK1/2 reduced systemin-mediated resistance to M. sexta herbivory (Fig. 5B). M. sexta larvae systemically induced MPK1, MPK2, and MPK3 activity in young tomato seedlings (Fig. 5A). Chewing insects generate other MAPK-activating signals in addition to systemin, such as mechanical signals (15) or fatty acid-amino acid conjugates (39). The corresponding signaling pathways all converge on MPK1, MPK2, and MPK3, which can explain why MPK1/2-silencing prevented a successful defense response against the attacking insect larvae. Overexpression of MPK1/2 orthologs or expression of active forms of their upstream MAPKK(K)s did not lead to JA synthesis (47) and had been shown to mimic pathogen-induced responses or confer resistance to microbial pathogens (43, 48–50). But no experiments were aimed at testing the performance of chewing insects on such plants. Because defense responses to pathogens and insects are often mutually exclusive, it is possible this crosstalk prevents MAPK gain-of-function plants from mounting a wound response. However, our reduction-of-function approach using TRV-VIGS revealed that MPK1, MPK2, and MPK3 not only function in pathogen-induced defenses but also are essential signaling components in the wound response that confers resistance to herbivorous insects. It remains to be determined how MAPKs that can be activated by multiple functionally diverse stress signals can signal stress-specific and mutually exclusive defense responses such as wounding- or pathogen-induced responses.
Materials and Methods
Plant Material and Growth Conditions.
Tomato plants (L. esculentum; alternative nomenclature Solanum lycopersicon) of the MicroTom or Castlemart variety were grown in AR66L growth chambers (Percival Scientific, Perry, IA) at 20°C under a 16-hr light [130 ± 20 μE m−2·s−1 (E = 1 mol of photons)] and 8-hr dark regime for optimal VIGS conditions. To increase PI protein synthesis, growth temperature was raised to 27°C 5 days before analysis. The transgene in 35S::prosys MicroTom plants had been backcrossed five times from Castlemart (34).
VIGS Constructs.
The TRV-based VIGS vectors pTRV1 and pTRV2 (30) were obtained from S. P. Dinesh-Kumar (Yale University, New Haven, CT). For details, see SI Text.
Infiltration of pTRV-Containing Agrobacterium tumefaciens Cultures into Cotyledons.
Eleven- to 12-day-old seedlings (true leaves just emerging) were infiltrated by vacuum infiltration with a mix of pTRV1- and pTRV2-carrying Agrobacteria according to Ekengren et al. (45). For syringe infiltrations, the same conditions were applied, but the cotyledons of young seedlings were infiltrated with Agrobacteria by using a 1-ml syringe.
sqRT-PCR.
See SI Text for details on sqRT-PCR.
MAPK Activity Assays.
Extracts from frozen leaf material were obtained and immunocomplex or in-gel kinase assays with myelin basic protein as an artificial MAPK substrate were carried out as described (14, 15). To measure MPK3 activity, magnesium in the kinase reaction buffer was substituted by manganese (26).
Radial Immunodiffusion Assay (RIDA).
PI-II protein levels in expressed leaf juice were measured as described (51). Anti-PI-II goat antiserum was generated by Spring Valley Laboratories (Woodbine, MD).
JA Analysis.
The upper (younger) four to five leaves of 4-week-old plants were left untreated or wounded by using a hemostat, and plants were incubated under standard conditions at 27°C. One hour after wounding, one-half of each wounded leaf or unwounded control leaf was excised, weighed, and frozen in liquid nitrogen for JA analysis. Typically, 300–500 mg of leaf material was collected from each plant. Samples were stored at −70°C. Twenty-four hours after wounding, PI-II protein levels in wounded and control plants were analyzed on the remaining portion of leaves by RIDA. The remaining leaf material was also used to determine MPK1/2 transcript levels by sqRT-PCR 3–4 days after collection of JA samples.
JA analysis using dihydro-JA as an internal standard was performed according to Schmelz et al. (52). For details, see SI Text.
MeJA Treatment.
pTRV-MPK1/2-infiltrated plants were exposed to MeJA (Sigma–Aldrich, St. Louis, MO) vapors (7 μl solved in 100 μl of ethanol and applied to cotton wicks) in 2-gallon sealed plastic bags for 12 hr under standard conditions at 27°C. Control plants were exposed to ethanol only. After an additional 12 hr, PI-II protein levels were analyzed by RIDA.
Tissue Sampling for sqRT-PCR, Kinase Assays, and RIDA.
Leaf samples for RNA analysis or in kinase assays were collected 4–6 weeks after infiltration. The development and extent of gene silencing were assessed by monitoring the photo-bleaching pattern of PHYTOENE DESATURASE-silenced plants according to Liu et al. (30). From each plant, 20–25 leaf discs from four or five young upper leaves were collected by using a hole puncher. Control (0-min) samples were collected and frozen immediately, whereas another 20–25 leaf discs were punched out in parallel from the same leaves and wounded by using serrated forceps. Wounded leaf discs were floated on MS medium (MS salts, 3% wt/vol sucrose, pH 5.8) for 10–15 min, quickly blotted dry on paper tissue, and frozen in liquid nitrogen for subsequent analysis by kinase assays. One-half of the control (0 min) samples was used for sqRT-PCR. The other half was used for immunocomplex- or in-gel kinase assays. Remaining tissue from these leaves was used for PI-II protein analysis by RIDA.
Herbivory Treatments.
M. sexta larvae (Carolina Biological Supply, Burlington, NC) were hatched and kept on an artificial diet for 3–5 days, after which they were placed on pTRV-MPK1/2- or pTRV-GFP-infiltrated plants. The average larval weight at this time was 3.8 ± 0.2 mg for experiment 1 and 5.6 ± 0.9 mg for experiment 2. One larva per plant was placed on the uppermost expanded leaf. Plants were incubated under standard conditions at 27°C. After 11 days, the entire foliage of almost all pTRV-MPK1/2-infiltrated plants was consumed and the experiment was stopped. Larval weight was determined, and the damaged plants were documented photographically. Herbivory-induced MPK activity was measured in 13- to 14-day-old L. esculentum var. Castlemart seedlings that displayed two unfolded leaves. M. sexta larvae (4th or 5th instar) were starved for 3 hr and then allowed to consume half of the terminal leaflet of the lower leaf. To avoid tissue damage by larval feet, larvae were manually placed close to the leaf edges, allowing them to access the leaf only with mandibles. After 3–5 min, the larvae were removed, and the wounded and unwounded leaves were frozen 10 min after onset of insect feeding for analysis by immunocomplex kinase assays.
Supplementary Material
Acknowledgments
We thank Sarah Refi for critically reading the manuscript, Charles R. Lovell (University of South Carolina) for allowing us to use his gas chromatograph, and George Matsui for advice on ethylene measurements. This research was supported by National Science Foundation Grant 0321453 (to J.W.S.), National Institutes of Health Grant R01GM57795 (to G.A.H.), and by grants from the University of South Carolina Magellan Scholar Program (S.S.P. and W.M.).
Abbreviations
- 35S::prosys
35S::prosystemin
- AOC
allene oxide cyclase
- AOS
allene oxide synthase
- JA
jasmonic acid
- LoxD
lipoxygenase D
- MeJA
methyl-JA
- PI
proteinase inhibitor
- sqRT-PCR
semiquantitative RT-PCR
- VIGS
virus-induced gene silencing
- TRV
tobacco rattle virus
- RIDA
radial immunodiffusion assay.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700344104/DC1.
References
- 1.Chen H, Wilkerson CG, Kuchar JA, Phinney BS, Howe GA. Proc Natl Acad Sci USA. 2005;102:19237–19242. doi: 10.1073/pnas.0509026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Constabel CP, Bergey DR, Ryan CA. Proc Natl Acad Sci USA. 1995;92:407–411. doi: 10.1073/pnas.92.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan CA. Biochim Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- 4.Pearce G, Strydom D, Johnson S, Ryan CA. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 5.McGurl B, Pearce G, Orozco-Cardenas ML, Ryan CA. Science. 1992;255:1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- 6.Orozco-Cardenas ML, McGurl B, Ryan CA. Proc Natl Acad Sci USA. 1993;90:8273–8276. doi: 10.1073/pnas.90.17.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GI, Howe GA. Plant J. 2003;33:567–576. doi: 10.1046/j.1365-313x.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- 8.Schilmiller AL, Howe GA. Curr Opin Plant Biol. 2005;8:369–377. doi: 10.1016/j.pbi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Li C, Lee GI, Howe GA. Proc Natl Acad Sci USA. 2002;99:6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratmann JW. Trends Plants Sci. 2003;8:247–250. doi: 10.1016/S1360-1385(03)00106-7. [DOI] [PubMed] [Google Scholar]
- 11.Wasternack C, Stenzel I, Hause B, Hause G, Kutter C, Maucher H, Neumerkel J, Feussner I, Miersch O. J Plant Physiol. 2006;163:297–306. doi: 10.1016/j.jplph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Higgins R, Lockwood T, Holley S, Yalamanchili R, Stratmann J. Planta. 2007;225:1535–1546. doi: 10.1007/s00425-006-0440-8. [DOI] [PubMed] [Google Scholar]
- 13.Schaller A, Oecking C. Plant Cell. 1999;11:263–272. doi: 10.1105/tpc.11.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holley SR, Yalamanchili RD, Moura SD, Ryan CA, Stratmann JW. Plant Physiol. 2003;132:1728–1738. doi: 10.1104/pp.103.024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratmann JW, Ryan CA. Proc Natl Acad Sci USA. 1997;94:11085–11089. doi: 10.1073/pnas.94.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutschmann F, Stalder U, Piotrowski M, Oecking C, Schaller A. Plant Physiol. 2002;129:156–168. doi: 10.1104/pp.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felix G, Boller T. Plant J. 1995;7:381–389. [Google Scholar]
- 18.O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- 19.Orozco-Cardenas M, Ryan CA. Proc Natl Acad Sci USA. 1999;96:6553–6557. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- 21.Farmer EE, Ryan CA. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narváez-Vásquez J, Florin-Christensen J, Ryan CA. Plant Cell. 1999;11:2249–2260. doi: 10.1105/tpc.11.11.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenzel I, Hause B, Maucher H, Pitzschke A, Miersch O, Ziegler J, Ryan CA, Wasternack C. Plant J. 2003;33:577–589. doi: 10.1046/j.1365-313x.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA. Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe GA, Lee GI, Itoh A, Li L, DeRocher AE. Plant Physiol. 2000;123:711–724. doi: 10.1104/pp.123.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayrose M, Bonshtien A, Sessa G. J Biol Chem. 2004;279:14819–14827. doi: 10.1074/jbc.M313388200. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Zhang S. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedley KF, Martin GB. J Biol Chem. 2004;279:49229–49235. doi: 10.1074/jbc.M410323200. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Xie Q-G, Smith-Becker J, Navarre DA, Kaloshian I. Mol Plant–Microbe Interact. 2006;19:655–664. doi: 10.1094/MPMI-19-0655. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Schiff M, Dinesh-Kumar SP. Plant J. 2002;31:777–786. doi: 10.1046/j.1365-313x.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 31.Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP. Plant J. 2004;39:734–746. doi: 10.1111/j.1365-313X.2004.02158.x. [DOI] [PubMed] [Google Scholar]
- 32.Howe GA, Ryan CA. Genetics. 1999;153:1411–1421. doi: 10.1093/genetics/153.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGurl B, Orozco-Cardenas ML, Pearce G, Ryan CA. Proc Natl Acad Sci USA. 1994;91:9799–9802. doi: 10.1073/pnas.91.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Jones AD, Howe GA. FEBS Lett. 2006;580:2540–2546. doi: 10.1016/j.febslet.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 35.Howe GA. J Plant Growth Regul. 2004;23:223–237. [Google Scholar]
- 36.Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 37.Seo S, Sano H, Ohashi Y. Plant Cell. 1999;11:289–298. doi: 10.1105/tpc.11.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo S, Katou S, Seto H, Gomi K, Ohashi Y. Plant J. 2007;49:899–909. doi: 10.1111/j.1365-313X.2006.03003.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu J, Hettenhausen C, Meldau S, Baldwin IT. Plant Cell. 2007;19:1096–1122. doi: 10.1105/tpc.106.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren D, Yang KY, Li GJ, Liu Y, Zhang S. Plant Physiol. 2006;141:1482–1493. doi: 10.1104/pp.106.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin H, Liu Y, Yang KY, Kim CY, Baker B, Zhang S. Plant J. 2003;33:719–731. doi: 10.1046/j.1365-313x.2003.01664.x. [DOI] [PubMed] [Google Scholar]
- 42.Sharma PC, Ito A, Shimizu T, Terauchi R, Kamoun S, Saitoh H. Mol Genet Genomics. 2003;269:583–591. doi: 10.1007/s00438-003-0872-9. [DOI] [PubMed] [Google Scholar]
- 43.Samuel MA, Ellis BE. Plant Cell. 2002;14:2059–2069. doi: 10.1105/tpc.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Jin H, Yang KY, Kim CY, Baker B, Zhang S. Plant J. 2003;34:149–160. doi: 10.1046/j.1365-313x.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 45.Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar SP, Martin GB. Plant J. 2003;36:905–917. doi: 10.1046/j.1365-313x.2003.01944.x. [DOI] [PubMed] [Google Scholar]
- 46.Li C, Williams MM, Loh Y-T, Lee GI, Howe GA. Plant Physiol. 2002;130:494–503. doi: 10.1104/pp.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, Hildebrand D, Sharp RE, Zhang S. Plant Cell. 2003;15:2707–2718. doi: 10.1105/tpc.011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 49.Del Pozo O, Pedley KF, Martin G. EMBO J. 2004;23:3072–3082. doi: 10.1038/sj.emboj.7600283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S, Liu Y. Plant Cell. 2001;13:1877–1889. doi: 10.1105/TPC.010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan CA. Anal Biochem. 1967;19:434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- 52.Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT. Plant J. 2004;39:790–808. doi: 10.1111/j.1365-313X.2004.02168.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.