Abstract
There are many cases of incongruence between phylogenetic hypotheses produced from morphological data and those produced from molecular data. In such instances, many researchers prefer to accept the results of molecular phylogenies. For example, in a recent analysis of primate phylogenies based on craniodental characters, Collard and Wood [Collard M, Wood BA (2000) Proc Natl Acad Sci USA 97:5003–5006] argued that, because craniodental data do not yield relationships concordant with molecular studies, the results of studies that employ such characters must be considered suspect. As most of our knowledge of mammalian evolution and phylogenetic history comes from craniodental fossils, these results have dramatic implications. However, the aforementioned analysis did not take into account the potentially confounding effects of allometry on quantitative craniodental characters. In this article, we employ a previously undescribed narrow allometric coding method that accounts for such confounding influences in phylogenetic analyses of craniodental morphology. By using essentially the same raw data set as Collard and Wood [Collard M, Wood BA (2000) Proc Natl Acad Sci USA 97:5003–5006], 65 quantitative craniodental characters were adjusted in a parsimony analysis for the primate tribe Papionini, a group of monkeys argued to display extensive homoplasy. The resulting phylogenetic tree was congruent with the phylogenetic tree based on molecular data for these species, thereby meeting the “criterion of congruence.” These results suggest that morphological data, when treated properly, can be considered as reliable as molecular data in phylogenetic reconstruction. Rather than accepting phylogenetic hypotheses from one data source over another, cases of incongruence should be examined with greater scrutiny.
Keywords: narrow allometry, character coding, papionins, cladistics, phylogeny
The relative efficacy of morphological and molecular data in phylogenetic analysis remains a major controversy in systematic biology. For example, phylogenetic analyses of molecular data favor the existence of the superordinal mammalian group Afrotheria (1–3), whereas initial analyses of morphological data did not (4–6). Molecular evidence favors a placement of Cetacea deep within Artiodactyla (1–3, 7–13), whereas most morphologically based phylogenies argue that Cetacea is the sister group to either artiodactyls or the extinct mesonychians (14–18). Finally, molecular and morphological data famously offer incongruent phylogenetic hypotheses regarding the placement of the mammalian order Chiroptera (2, 18–22). When such inconsistencies exist, the value of morphological (particularly osteological) data is often questioned because of their perceived propensity for homoplasy (23) and the inconsistency of their phylogenetic results (24).
Within the order Primates, there are also a few well documented cases of apparent incompatibility between phylogenetic hypotheses generated from molecular and morphological data. In recent years, probably the most widely cited examples concern the extant hominoid apes and the extant cercopithecine monkey tribe Papionini. Specifically, Collard and Wood (25, 26) have demonstrated that phylogenetic hypotheses generated from craniodental morphology are inconsistent with those generated from molecular data for these primate groups. Accepting the results of the molecular studies, these authors conclude that such morphological characters are unreliable for reconstructing phylogeny. Because our understanding of primate and, more broadly, mammalian evolution is based on a fossil record composed largely of craniodental material, the implications of these studies are especially dramatic for phylogenetic hypotheses that include fossil taxa.
The issues raised by Collard and Wood (25, 26) are significant. However, the analyses performed by them are not without issue, and some of these problems have been identified (27, 28). Thus, the choice of outgroup(s) and of which taxa to include can have significant effects on character polarities, and these decisions will influence the resulting phylogenetic trees. In the case of hominoid phylogeny, a recent study (28) recovered the molecular cladogram (i.e., that deemed most accurate by many researchers) by simply expanding the outgroup and including hominin fossils that helped to break down the “long branch” that separates Homo from the other apes.
Additionally, as noted by Jolly (27), the size correction method used in the Collard and Wood studies (25, 26) does not properly adjust for shape changes that are correlated with size (i.e., allometry). The geometric mean method of size correction is isometric and equalizes specimen volumes while maintaining their shapes (29), but it does not account for shape differences that are correlated with size. Therefore, every quantitative craniometric character that is allometrically influenced is simply grouping taxa on the basis of shape correlated with body size. These characters violate the assumption of character independence that is critical in phylogenetic analysis. In the face of pervasive allometries, body size is being coded multiple times in the analyses, and this repetition no doubt has a strong influence on their resulting trees. A subsequent study attempted to use a different method of size correction, namely regression analysis with the retention of residuals (30). However, as has been demonstrated (29, 31), this method is undesirable because residuals eliminate not only size but also most shape information as well.
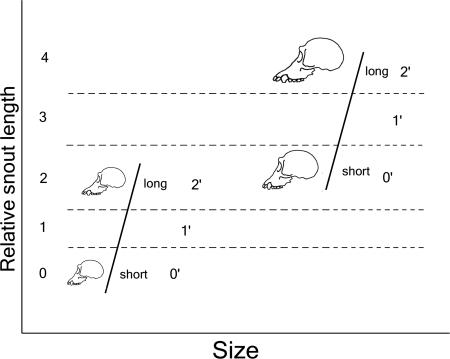
It is difficult to adequately control for differences in body size without losing phylogenetically meaningful information. One option is to simply exclude any characters that are identified as being allometrically influenced, but this technique would result in major loss of useful information because so much of form is correlated with size (32). Even when a character is significantly correlated with body size, taxa of similar sizes may have different morphologies that are of phylogenetic value. For example, when taxa form two distinct size groups, small species as well as large species may have relatively long snouts, and although these relatively long snouts may be homologous, these homologies would be obscured by most methods of character coding that attempt to correct for size (Fig. 1). In such cases, a “narrow allometry” approach (33) would be better suited to detect similarities in morphology, but no character coding method of this sort has been devised until now. The need for better methods in treating quantitative morphological characters has been noted (25), and we believe that the method developed here represents such an improvement.
Fig. 1.
Heuristic comparison of narrow allometric coding and conventional coding of size-adjusted data for hypothetical character “relative snout length.” The study group exhibits positive allometry for the character relative snout length, and taxa within the group fall into two basic size groups, small and large. The black lines represent ranges of relative snout length within each size group. Conventional conversion of craniometric data into phylogenetic characters (depicted in dashed lines and numbers on the y axis) would divide the entire range of relative snout lengths (the y axis) into segments horizontally, in this case producing five states. Our method of narrow allometric coding (depicted by diagonally arranged numbers marked with a prime) performs the same segmenting procedure but applies it to the two size groups separately such that the shortest-snouted species in each group are coded as “short,” and the longest-snouted species in each group are coded as “long.”
Accordingly, the purpose of the present study is twofold. First, we investigate the extent to which the craniometric characters of Collard and Wood's papionin data set were influenced by body size. We chose to focus on the papionin (rather than hominoid) data because allometry seems to have had a strong effect on the overall form of crania in this clade (34–38). We then generate a phylogenetic hypothesis for African papionins [using essentially the same craniodental data set as the Collard and Wood studies (25, 26)] that employs a narrow allometric approach to coding allometrically influenced characters (see Materials and Methods). If any of the size-adjusted characters are significantly correlated with body size, then this method should provide a more meaningful portrayal of the craniometric data as character states. Should it also produce results that are more congruent with the molecular phylogenies, such an outcome may be reassuring to morphological systematists.
Results
Supporting information (SI) Table 3 lists the characters in this study determined to be allometrically influenced. Thirty of the 65 craniodental characters in this study were significantly affected by allometry. Over one-third of these allometrically influenced characters (eleven) were concentrated in the cranial vault and base.
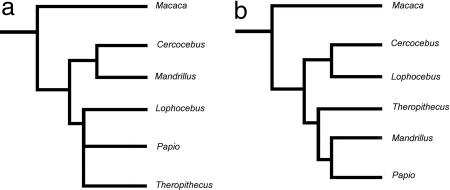
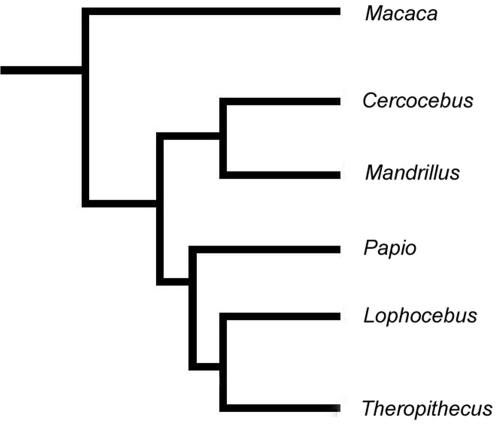
For comparison, the molecular phylogeny for the extant papionins, as well as the most parsimonious tree derived from the original Collard and Wood (25) data set, is presented in Fig. 2. The resulting cladogram from the current analysis is provided in Fig. 3 and summarized with tree statistics in Table 1. By using the narrow allometric coding method, the most parsimonious tree from our analysis was congruent with the molecular phylogenetic tree (Table 1 and Fig. 3). Bootstrap support for a Cercocebus/Mandrillus clade was highest at 84%. Bootstrap support for a Papio/Lophocebus/Theropithecus clade was 58%, and bootstrap support for a Lophocebus/Theropithecus clade was 52%.
Fig. 2.
Previous hypotheses of papionin phylogenetic relationships. (a) Hypothesized phylogenetic tree of the extant Papionini from molecular (mtDNA and Y chromosome) data (70–74) compared with b, the most parsimonious tree derived from the craniodental data set of Collard and Wood (25, 26), using Macaca rather than Pan as the outgroup.
Fig. 3.
Most parsimonious phylogenetic tree of the extant Papionini from craniodental morphological data in the mixed-sex analysis. Note that this tree is congruent with the hypothesized phylogenetic tree for the extant papionins from molecular data in Fig. 2a.
Table 1.
Summary of most parsimonious phylogenetic trees produced from an exhaustive search in PAUP 4.10 with bootstrap support for various clades
| Analysis | Tree length | CI | RI | RC | HI | Bootstrap support, % |
||
|---|---|---|---|---|---|---|---|---|
| Cercocebus/Mandrillus | Papio/Lophocebus/Theropithecus | Lophocebus/Theropithecus | ||||||
| Mixed-sex | 208 | 0.62 | 0.41 | 0.26 | 0.38 | 84 | 58 | 52 |
RC, rescaled consistency index; HI, homoplasy index.
Discussion
The results of the analysis presented here suggest that, when size and allometry are accounted for in a biologically meaningful way, phylogenetic analysis of craniodental data can produce trees that are congruent with those derived from molecular data. If such congruence is considered to be a test of the reliability of morphological data, as maintained by many (25, 26, 39, 40), then craniodental data would seem to be perfectly suitable for phylogenetic analysis of extant as well as extinct taxa.
Whereas the “criterion of congruence” is argued by many authors to be a prerequisite for the acceptance of morphologically based phylogenies, we do not believe that such a test is necessarily appropriate. As others have argued (28), insistence that morphological data must be able to recover molecular trees before being applied to extinct taxa neglects the well documented fact that the inclusion of fossil taxa in phylogenetic analysis can increase overall phylogenetic accuracy (41, 42) by providing unique morphologies that help to refine assessments of character transformation (43–47). In other words, fossil data are a strongpoint of morphological phylogenetics, and testing the latter without the former is at best conservative, and at worst unjustified.
Moreover, we see no logical basis for assuming that molecular trees constitute an unassailable phylogenetic framework against which other methods or types of data can be tested. When molecular and morphological data disagree, it should inspire scrutiny of both. In molecular systematics, taxon and gene sampling can often be improved, and the effect of different tree-building methods can be explored. Morphologists can re-evaluate their data by employing a battery of primary tests of homology (e.g., comparing the ontogeny of characters), by expanding their taxonomic sample, and by adding new fossil material, to enumerate just a few approaches. Such refinements have often brought the two closer to agreement. Recently discovered fossil evidence provides morphological support for the Cetacea–hippopotamid clade (48), and renewed investigation of Afrotherian morphology has uncovered support here as well (49, 50). When such congruence has been achieved, we contend that it is the phylogenetic hypothesis that has passed a test, not the morphological data. In the present case, we have demonstrated that craniodental morphology does support a Cercocebus/Mandrillus clade, and a Papio/Lophocebus/Theropithecus clade. In our view, the congruence of our results with those of molecular analyses provides strong support for their shared phylogenetic hypothesis.
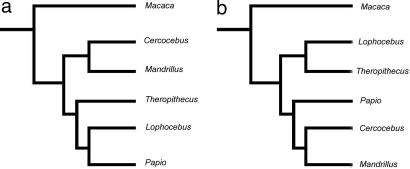
Because papionins are a highly sexually dimorphic group with drastically different male and female morphologies, some may argue that it is less than ideal to average these morphologies together to create what is, in essence, an “imaginary” morphotype. With this point in mind, we decided to further investigate the data by analyzing the sexes separately. Whereas some previous authors have suggested that papionin males may be better suited for detailing differences in anatomy (51–54), a number of studies commonly suggest that males are more variable in many features (55–59) and, by extension, probably less valuable in taxonomic and phylogenetic studies. Instead, many authors commonly use females as the optimal phenotype for interspecific analysis (60, 61). The results of the sex-specific analyses are presented in Fig. 4 and Table 2.
Fig. 4.
Most parsimonious phylogenetic trees of the extant Papionini from craniodental data in the male analysis (a) and from craniodental data in the female analysis (b).
Table 2.
Summary of most parsimonious phylogenetic trees produced from sex-specific exhaustive searches in PAUP 4.10b with bootstrap support for various clades
| Analysis | Tree length | CI | RI | RC | HI | Bootstrap support, % |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cercocebus/Mandrillus | Papio/Lophocebus/Theropithecus | Papio/Cercocebus/Mandrillus | Papio/Lophocebus | Lophocebus/Theropithecus | ||||||
| Males | 193 | 0.67 | 0.49 | 0.33 | 0.33 | 99 | 91 | — | 86 | — |
| Females | 208 | 0.62 | 0.41 | 0.26 | 0.38 | 69 | — | 75 | — | 50 |
Abbreviations are as in Table 1.
Given the previous debate, it is noteworthy that papionin males rather than females produce a tree congruent with both the mixed-sex analysis and the molecular tree in suggesting Cercocebus/Mandrillus and Papio/Lophocebus/Theropithecus clades. In addition, the male analysis displays a shorter tree, higher CI, RI, and RC statistics, and higher bootstrap values (Table 2). Taken together, these statistics indicate that the male craniodental data are better at detecting actual versus apparent synapomorphies and produce more stable trees. Similar results were recently obtained by morphometric analysis of hominoid temporal bones (62). In a highly sexually dimorphic group such as the Papionini, it is perhaps not surprising that the larger and more exaggerated morphologies of males are potentially more useful in reconstructing phylogeny, as suggested by Fleagle and McGraw (54). We would also suggest that the exaggerated male morphologies are due in part to sexual selection. As sexually selected traits are no doubt closely tied to mating preferences and mate recognition systems (63), it is probable that more closely related taxa shared a similar mate recognition system in the recent past that would produce similar sexually selected character states in males. Whatever the underlying reason, the performance of male papionins suggests that phylogenetic studies of highly sexually dimorphic primates should take particular note of male morphologies in the future. At the very least, they should not be ignored as aberrant or too variable for analysis.
One final point should be made regarding the composition of the data set used here. The 62 characters from Collard and Wood (25, 26), as well as the two characters from Fleagle and McGraw (54, 64), consisted of craniometric measurements only. We certainly accept that such characters are valid data to be used in phylogenetic analyses. However, size-adjusted linear measurements are not the only characters likely to be phylogenetically informative. There are many potentially informative features of the skull and dentition that are not conducive to simple linear measurement, such as the presence or absence and shape of certain fossae or foramina. In fact, one recent analysis has identified multiple qualitative craniodental characters among the papionins that unite the same clades found here (65).
Whereas the craniometric data set is advantageous in its objectivity and reproducibility, it does not capture as much phylogenetic information as a combined quantitative and traditional qualitative character analysis. Future primate phylogenetic analyses should attempt to combine both types of morphological characters, finding ways to quantify the latter when possible (66). Given the present results, we also suggest that a narrow allometric coding approach such as the one outlined here should be used when the size distribution of taxa permits. Finally, we believe that the present study demonstrates that it remains more beneficial to focus attention on how to treat morphological data than on whether morphological characters are “better” or “worse” sources of information.
Materials and Methods
The data set used by Collard and Wood (25, 26) for the extant papionins was kindly provided by Mark Collard. Taxa, sample sizes, and a character list are given in SI Tables 3 and 4. Because our coding method is based on narrow allometries, Pan is not an appropriate outgroup because the allometric trajectories influencing its craniodental morphology are not comparable with those observed in papionin monkeys. For our purposes, such a phylogenetically and phenetically distant taxon cannot be used (62, 67). Given that Macaca is universally accepted as the sister group of the other papionins considered here (68–74), it is assigned as the outgroup for all analyses. To render the original results of Collard and Wood (25, 26) more directly comparable with our own, their original 62-character data set was reanalyzed excluding Pan and assigning Macaca as the outgroup, and without using the narrow allometric coding method. The resulting tree is presented in Fig. 2b. Recoding of this matrix using weighted gap coding and including the three characters added in our analysis also yields the tree shown in Fig. 2b.
The coding method we employ here is applicable only to clades that contain more or less discrete body size clusters. Clades that contain taxa spanning a wide and continuous range of body sizes present additional problems that our methodology is unequipped to address. However, for clades such as the papionins, within which taxa can be grouped into two or more discontinuous size categories, the method outlined here should prove useful. A flow chart summarizing the narrow allometric coding method used in this study is presented in SI Fig. 5.
Sixty-five characters were used for the analysis: the 62 quantitative characters from Collard and Wood (25, 26), two additional quantitative characters noted more recently by Fleagle and McGraw (54, 66), and a body size character (explained further below). As an isometric size correction, linear measurements for each specimen were divided by the geometric mean of all measurements for that specimen§. After this isometric size correction, the resulting values for each character represented some aspect of “shape” (75, 76).
By definition, allometrically influenced characters are those whose shape is significantly correlated with size (77). To determine which characters were allometrically influenced, Pearson correlation analyses of all isometrically size-adjusted shape characters against the geometric mean of all cranial measurements were performed. Theoretically, these correlations should be run for each character for each specimen against the geometric mean of all cranial measurements for that specimen. This approach has the advantage of providing more data points to detect significance in the correlations, especially if the phylogenetic analysis in question involves a small number of taxa. However, this approach also has the serious disadvantage of being overly sensitive to characters that have a small but statistically significant size-correlated component. The more general approach used here instead uses the average size-adjusted value for each character for each taxon and then correlates these values to the average geometric mean for each taxon (i.e., the geometric means of all specimens within that taxon averaged together).
To illustrate by example, if there are size-adjusted measurements for character X on 10 specimens of species Y, the average value of character X and the average geometric mean for species Y were used in the correlation analyses. Males and females were treated separately in the correlation analysis so that there were two data points per taxon. The lowest significant r value produced from these analyses was 0.598, which is an acceptably high correlation coefficient. We suggest that correlation values <0.500 are probably not biologically significant enough to warrant application of the narrow allometric coding procedure outlined here.
After the correlation analyses were performed, those shape characters that were significantly correlated with the geometric mean of all cranial measurements (size) were determined to be, by definition, significantly influenced by allometry (see SI Table 3 for the complete list of allometrically influenced characters). Because of their correlation with body size, these characters are not independent characters and are not suitable for phylogenetic analysis without some sort of character correction. For these characters, a previously undescribed coding procedure that uses a narrow allometric approach was used to disentangle the effects of allometry.
Following Collard and Wood (25), male and female character values were averaged for each taxon in the analysis. To correct for allometrically influenced characters during coding, the extant papionins were then divided into “large” and “small” size categories. This division involves no phylogenetic assumption; these are size-based groups, not presumed clades. Macaques (Macaca) and mangabeys (Cercocebus and Lophocebus) were considered small taxa whereas baboons (Papio), geladas (Theropithecus), mandrills, and drills (Mandrillus) were considered large taxa (78). In the case of papionins, the two size groups are fairly obvious, but more rigorous methods for identifying size groups may be required for other taxa. After operational taxonomic unit assignment to the large or small size category, character states for each character were then coded by comparing taxa within their size category, thereby “naturally” controlling for allometric changes in shape (i.e., a narrow allometric approach). For example, if a hypothetical character such as “snout length” has been determined to be allometrically influenced using the procedure outlined above, the taxon with the shortest relative snout length in the “small” category would be coded as “short,” and the taxon with the shortest relative snout length in the “large” category would also be coded as “short” (see Fig. 1). The result is that a taxon in the small category can have the same character state as a taxon in the large category.
For all 64 quantitative characters, weighted gap coding was used (78). Because only six taxa were used in the analysis and only three taxa were assigned to each size category for allometrically influenced characters, we chose to divide each quantitative character into three character states. Some studies recommend that quantitative characters should be divided into as many states as possible for phylogenetic analysis (e.g., 32 states as in refs. 79 and 80), but this approach excessively increases the weight of these characters relative to characters that are coded with other methods (body size in this case). Moreover, using the maximum number of states available in a software package is an arbitrary choice because one can choose to use different software. Alternatively, a nonarbitrary solution is to use the number of taxa to be coded as the number of states (three in this case), because this is the minimum number of states needed to give the taxa the opportunity to each have a unique state.
A final nonquantitative character, body size, was coded according to the size categories named above: 0 = small, 1 = large. This character was included because, by using the coding procedure outlined above, the effects of body size were removed from all of the other characters. Whereas previous analyses may have been biased by including too many characters that are correlated with body size, we did not want this analysis to be biased in the opposite direction by excluding body size completely.
After character coding, the mixed-sex matrix (SI Table 5) was subjected to parsimony analysis in PAUP 4.10b (81). All characters were ordered, and an exhaustive search was used to identify the minimum length tree. To provide confidence intervals for the support of each clade produced by our analysis (82), a phylogenetic bootstrap was performed by using a branch-and-bound search routine, 10,000 replications, and sampling with replacement.
Supplementary Material
Acknowledgments
We thank Mark Collard (University of British Columbia, Vancouver, BC, Canada) for providing the raw measurements used in the original Collard and Wood analyses. Fred Grine, Bill Jungers, John Fleagle, David Strait, and two anonymous reviewers all provided helpful comments and criticisms on this manuscript. We thank John Wiens, Maureen O'Leary, James Rohlf, and Bill Jungers for insightful comments and instruction on character coding, allometry, and correlation analyses. This work was generously supported in part by a grant from the L. S. B. Leakey Foundation, as well as a Graduate Council Fellowship provided by Stony Brook University (to C.C.G.).
Abbreviations
- RI
retention index
- CI
consistency index.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702174104/DC1.
Because the upper and lower premolar indices used by Fleagle and McGraw (54, 64) are already size-adjusted, they were not divided by the geometric mean as a size-adjustment. The indices calculated by Fleagle and McGraw (54, 64) were not included in the calculation of the geometric mean for each specimen.
References
- 1.Madsen O, Scally M, Douady CJ, Kao DJ, DeBry RW, Adkins R, Amrine HM, Stanhope MJ, de Jong WW, Springer MS. Nature. 2001;409:610–614. doi: 10.1038/35054544. [DOI] [PubMed] [Google Scholar]
- 2.Murphy WJ, Eizirik E, O'Brien SJ, Madsen O, Scally M, Douady CJ, Teeling E, Ryder OA, Stanhopw MJ, de Jong WW, Springer MS. Science. 2001;294:2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- 3.Springer MS, Murphy WJ, Eizirik E, O'Brien SJ. Proc Natl Acad Sci USA. 2003;100:1056–1061. doi: 10.1073/pnas.0334222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prothero DR, Manning EM, Fischer M. The Phylogeny and Classification of the Tetrapods. In: Benton MJ, editor. Mammals. Vol 2. Oxford: Clarendon Press; 1988. pp. 201–234. [Google Scholar]
- 5.Thewissen JGM, Domning DP. J Vertebr Paleontol. 1992;12:494–504. [Google Scholar]
- 6.Fischer MS, Tassy P. Mammal Phylogeny. In: Szalay FS, Novacek MJ, McKenna MC, editors. Placentals. New York: Springer; 1993. pp. 217–234. [Google Scholar]
- 7.Springer MS, Kirsch JAW. J Mammal Evol. 1993;1:149–166. [Google Scholar]
- 8.Graur D, Higgins DG. Mol Biol Evol. 1994;11:357–364. doi: 10.1093/oxfordjournals.molbev.a040118. [DOI] [PubMed] [Google Scholar]
- 9.Gatesy J, Hayashi C, Cronin MA, Arctander P. Mol Biol Evol. 1996;13:954–963. doi: 10.1093/oxfordjournals.molbev.a025663. [DOI] [PubMed] [Google Scholar]
- 10.Gatesy J, Milinkovitch M, Waddell V, Stanhope M. Syst Biol. 1999;48:6–20. doi: 10.1080/106351599260409. [DOI] [PubMed] [Google Scholar]
- 11.Gatesy J. Mol Biol Evol. 1997;14:537–543. doi: 10.1093/oxfordjournals.molbev.a025790. [DOI] [PubMed] [Google Scholar]
- 12.Ursing BM, Arnason U. Proc R Soc London Ser B. 1998;265:2251–2255. doi: 10.1098/rspb.1998.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikaido M, Rooney AP, Okada N. Proc Natl Acad Sci USA. 1999;96:10261–10266. doi: 10.1073/pnas.96.18.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Leary MA. In: The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea. Thewissen JGM, editor. New York: Plenum; 1998. pp. 133–161. [Google Scholar]
- 15.Luo ZX, Gingerich PD. Univ Michigan Papers Paleontol. 1999;31:1–98. [Google Scholar]
- 16.O'Leary MA, Geisler JH. Syst Biol. 1999;48:455–490. doi: 10.1080/106351599260102. [DOI] [PubMed] [Google Scholar]
- 17.Geisler JH. Am Mus Nov. 2001;40:1–53. [Google Scholar]
- 18.Thewissen JGM, Williams EM, Roe LJ, Hussain ST. Nature. 2001;413:277–281. doi: 10.1038/35095005. [DOI] [PubMed] [Google Scholar]
- 19.Wible JR, Novacek MJ. Am Mus Novitates. 1988;2911:1–19. [Google Scholar]
- 20.Beard KC. Mammal Phylogeny. In: Szalay FS, Novacek MJ, McKenna MC, editors. Placentals. New York: Springer; 1993. pp. 129–150. [Google Scholar]
- 21.Douady CJ, Chatelier PI, Madsen O, de Jong WW, Catze F, Springer MS, Stanhope MJ. Mol Phylogenet Evol. 2002;25:200–209. doi: 10.1016/s1055-7903(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 22.Simmons NB. In: The Rise of Placental Mammals. Rose KD, Archibald JD, editors. Baltimore: Johns Hopkins Univ Press; 2005. pp. 159–174. [Google Scholar]
- 23.Lockwood CA, Fleagle JG. Yearb Phys Anthropol. 1999;42:189–232. doi: 10.1002/(sici)1096-8644(1999)110:29+<189::aid-ajpa7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Pilbeam DR. Proc Natl Acad Sci USA. 2000;97:10684–10686. doi: 10.1073/pnas.210390497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collard M, Wood BA. Proc Natl Acad Sci USA. 2000;97:5003–5006. doi: 10.1073/pnas.97.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collard M, Wood BA. J Hum Evol. 2001;41:167–194. doi: 10.1006/jhev.2001.0487. [DOI] [PubMed] [Google Scholar]
- 27.Jolly CJ. Yearb Phys Anthropol. 2001;44:177–204. doi: 10.1002/ajpa.10021. [DOI] [PubMed] [Google Scholar]
- 28.Strait DS, Grine FE. J Hum Evol. 2004;47:399–452. doi: 10.1016/j.jhevol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Jungers WL, Falsetti AB, Wall CE. Yearb Phys Anthropol. 1995;38:137–161. [Google Scholar]
- 30.Nadal-Roberts M, Collard M. Folia Primatol. 2005;76:207–221. doi: 10.1159/000086022. [DOI] [PubMed] [Google Scholar]
- 31.Bookstein FL. Syst Zool. 1989;38:173–180. [Google Scholar]
- 32.Lycett SJ, Collard M. J Hum Evol. 2005;49:618–642. doi: 10.1016/j.jhevol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Smith RJ. Am J Physiol. 1984;246:R152–R160. doi: 10.1152/ajpregu.1984.246.2.R152. [DOI] [PubMed] [Google Scholar]
- 34.Freedman L. Growth. 1962;26:117–128. [PubMed] [Google Scholar]
- 35.Collard M, O'Higgins P. Evol Dev. 2001;3:322–331.36. doi: 10.1046/j.1525-142x.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- 36.Frost S, Marcus L, Bookstein F, Reddy D, Delson E. Anat Rec A. 2003;275A:1048–1972. doi: 10.1002/ar.a.10112. [DOI] [PubMed] [Google Scholar]
- 37.Singleton M. J Hum Evol. 2002;42:547–578. doi: 10.1006/jhev.2001.0539. [DOI] [PubMed] [Google Scholar]
- 38.Leigh SR, Shah NS, Buchanan LS. J Hum Evol. 2003;45:285–316. doi: 10.1016/j.jhevol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Gibbs S, Collard M, Wood B. Proc Natl Acad Sci USA. 2000;97:11130–11132. doi: 10.1073/pnas.190252697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilbeam DR. Mol Phylog Evol. 1996;5:155–168. doi: 10.1006/mpev.1996.0010. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler WC. In: Extinction & Phylogeny. Novacek MJ, Wheeler QD, editors. New York: Columbia Univ Press; 1992. pp. 205–215. [Google Scholar]
- 42.Zwickl DJ, Hillis DM. Syst Biol. 2002;51:588–598. doi: 10.1080/10635150290102339. [DOI] [PubMed] [Google Scholar]
- 43.Gauthier J, Kluge A, Row T. Cladistics. 1988;4:105–209. doi: 10.1111/j.1096-0031.1988.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 44.Donoghue MJ, Doyle JA, Gauthier J, Kluge AG, Rowe T. Ann Rev Ecol Syst. 1989;20:431–460. [Google Scholar]
- 45.Gatesy J, O'Leary MA. Trends Ecol Evol. 2001;16:562–570. [Google Scholar]
- 46.Gatesy J, Amato G, Norell M, DeSalle R, Hayashi C. Syst Biol. 2003;52:403–422. doi: 10.1080/10635150390197037. [DOI] [PubMed] [Google Scholar]
- 47.Springer MS, Teeling EC, Madsen O, Stanhope MJ, de Jong WW. Proc Natl Acad Sci USA. 2001;98:6241–6246. doi: 10.1073/pnas.111551998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geisler JH, Uhen M. J Vert Paleontol. 2003;23:991–996. [Google Scholar]
- 49.Seiffert ER. Durham, NC: Duke Univ; 2003. PhD thesis. [Google Scholar]
- 50.Werdelin L, Nilssone A. J Theor Biol. 1999;196:61–72. doi: 10.1006/jtbi.1998.0821. [DOI] [PubMed] [Google Scholar]
- 51.Nakatsukasa M. Z Morph Anthropol. 1994a;80:125–136. [Google Scholar]
- 52.Nakatsukasa M. Afr Study Monogr. 1994b;21:1–61. [Google Scholar]
- 53.Nakatsukasa M. Folia Primatol. 1996;66:15–24. doi: 10.1159/000157181. [DOI] [PubMed] [Google Scholar]
- 54.Fleagle JG, McGraw WS. J Hum Evol. 2002;42:267–292. doi: 10.1006/jhev.2001.0526. [DOI] [PubMed] [Google Scholar]
- 55.Wood BA. J Zool. 1976;180:15–34. [Google Scholar]
- 56.Wood BA, Li Y, Willoughby C. J Anat. 1991;174:185–205. [PMC free article] [PubMed] [Google Scholar]
- 57.Leutenegger W, Cheverud J. Int J Primatol. 1982;3:387–402. [Google Scholar]
- 58.Leutenegger W, Cheverud J. In: Size and Scaling in Primate Biology. Jungers WL, editor. New York: Plenum; 1985. pp. 32–50. [Google Scholar]
- 59.Albrecht GH, Gelvin BR, Miller JMA. In: Gorilla Biology. Taylor AB, Goldsmith ML, editors. Cambridge, UK: Cambridge Univ Press; 2003. pp. 62–103. [Google Scholar]
- 60.Gaulin SJC, Sailer LD. Am Anthropol. 1985;87:111–119. [Google Scholar]
- 61.Nunn CL, Barton RA. Evol Anthropol. 2001;10:81–96. [Google Scholar]
- 62.Lockwood CA, Kimbel WH, Lynch JM. Proc Natl Acad Sci USA. 2004;101:4356–4360. doi: 10.1073/pnas.0306235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paterson HEH. In: Species and Speciation. Vrba ES, editor. 1985. pp. 21–29. Transvaal Museum Monograph 4. [Google Scholar]
- 64.Fleagle JG, McGraw WS. Proc Natl Acad Sci USA. 1999;96:1157–1161. doi: 10.1073/pnas.96.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilbert CC. J Hum Evol. 2007;53:69–102. doi: 10.1016/j.jhevol.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Seiffert ER, Kappelman J. J Hum Evol. 2001;40:301–318. doi: 10.1006/jhev.2000.0455. [DOI] [PubMed] [Google Scholar]
- 67.Gaffney ES. In: Phylogenetic Analysis and Paleontology. Cracraft J, Eldredge N, editors. New York: Columbia Univ Press; 1979. pp. 79–112. [Google Scholar]
- 68.Szalay FS, Delson E. Evolutionary History of the Primates. New York: Academic; 1979. [Google Scholar]
- 69.Strasser E, Delson E. J Hum Evol. 1987;16:81–99. [Google Scholar]
- 70.Disotell TR, Honeycutt RL, Ruvulo M. Mol Biol Evol. 1992;9:1–13. doi: 10.1093/oxfordjournals.molbev.a040700. [DOI] [PubMed] [Google Scholar]
- 71.Disotell TR. Am J Phys Anthropol. 1994;94:47–57. doi: 10.1002/ajpa.1330940105. [DOI] [PubMed] [Google Scholar]
- 72.Disotell TR. In: Old World Monkeys. Whitehead PF, Jolly CJ, editors. Cambridge, UK: Cambridge Univ Press; 2000. pp. 29–56. [Google Scholar]
- 73.Harris EE, Disotell TR. Mol Biol Evol. 1998;15:892–900. doi: 10.1093/oxfordjournals.molbev.a025993. [DOI] [PubMed] [Google Scholar]
- 74.Tosi AJ, Disotell TR, Morales JC, Melnick DJ. Mol Phyl Evol. 2003;27:510–521. doi: 10.1016/s1055-7903(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 75.Mosimann JE. J Am Stat Assoc. 1970;56:930–945. [Google Scholar]
- 76.Darroch JN, Mosimann JE. Biometrika. 1985;72:241–252. [Google Scholar]
- 77.Mosimann JE, James FC. Evolution (Lawrence, Kans) 1979;23:444–459. doi: 10.1111/j.1558-5646.1979.tb04697.x. [DOI] [PubMed] [Google Scholar]
- 78.Bernstein RM, Leigh SR, Donovan SM, Monaco MH. Am J Phys Anthropol. 2007;132:247–260. doi: 10.1002/ajpa.20521. [DOI] [PubMed] [Google Scholar]
- 79.Thiele K. Cladistics. 1993;9:275–304. doi: 10.1111/j.1096-0031.1993.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 80.Poe S, Wiens JJ. In: Phylogenetic Analysis of Morphological Data. Wiens JJ, editor. Washington, DC: Smithsonian Institution Press; 2000. pp. 20–36. [Google Scholar]
- 81.Swofford D. PAUP: Phylogenetic Analysis Using Parsimony. Sunderland, MA: Sinauer; 2001. Version 4.0b10. [Google Scholar]
- 82.Felsenstein J. Evolution (Lawrence, Kans) 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.