Abstract
Women carrying germ-line mutations in BRCA1 are strongly predisposed to developing breast cancers with characteristic features also observed in sporadic basal-like breast cancers. They appear as high-grade tumors with high proliferation rates and pushing borders. On the molecular level, they are negative for hormone receptors and ERBB2, display frequent TP53 mutations, and express basal epithelial markers. To study the role of BRCA1 and P53 loss of function in breast cancer development, we generated conditional mouse models with tissue-specific mutation of Brca1 and/or p53 in basal epithelial cells. Somatic loss of both BRCA1 and p53 resulted in the rapid and efficient formation of highly proliferative, poorly differentiated, estrogen receptor-negative mammary carcinomas with pushing borders and increased expression of basal epithelial markers, reminiscent of human basal-like breast cancer. BRCA1- and p53-deficient mouse mammary tumors exhibit dramatic genomic instability, and their molecular signatures resemble those of human BRCA1-mutated breast cancers. Thus, these tumors display important hallmarks of hereditary breast cancers in BRCA1-mutation carriers.
Keywords: mouse models, conditional knockout
Germ-line mutations in the human breast cancer susceptibility gene BRCA1 are responsible for 40% to 50% of hereditary breast cancers and confer increased risk for development of ovarian, colon, and prostate cancers (1, 2). BRCA1 has been implicated in various cellular processes, including maintenance of genome integrity, DNA replication and repair, chromatin remodeling, and transcriptional regulation (3, 4). Although the exact mechanism of mammary tumor suppression by BRCA1 remains largely unknown, cells with dysfunctional BRCA1 show defects in survival and proliferation, increased radiosensitivity, chromosomal abnormalities, G2/M checkpoint loss, and impaired homologous recombination repair (5).
BRCA1-mutated breast cancers that arise in women with germ-line mutations in BRCA1 are high-grade, hormone receptor-negative breast carcinomas with frequent mutation of TP53 (4, 6). They also possess a basal-like phenotype as defined by the expression of markers that are typical for basal/myoepithelial cells, such as the basal cytokeratins (CKs) CK5/6 and CK14 (7). Indeed, strong molecular similarities are observed between hereditary BRCA1-mutated breast cancers and sporadic basal-like breast carcinomas (8, 9). This phenotypic overlap has led to the hypothesis that sporadic basal-like cancers may have defects in BRCA1-related pathways, such as the amplification of EMSY and the methylation of BRCA1 and FANCF (10).
Despite the fact that several mouse strains with conventional or conditional mutations in Brca1 have been generated (11), no good mouse model for BRCA1-mutated basal-like breast cancer has been developed so far. Most conventional Brca1 knockouts are embryonic-lethal when bred to homozygosity, yet heterozygous Brca1 female mice are not tumor-prone (12–14). Hypomorphic Brca1tr/tr and Brca1Δ11/Δ11; p53+/− mutant mice, as well as conditional MMTVcre; Brca1Co/Co and MMTVcre; Brca1Co/Co; p53+/− mice develop mammary tumors with diverse histological patterns (15–17). Moreover, Cre-mediated recombination of the most widely used conditional Brca1 allele (Brca1Co) (18) generates a hypomorphic Brca1Δ11 allele, which encodes BRCA1-Δ11, a naturally occurring splice variant of Brca1 (19). Mouse mammary tumor models based on conditional Brca1-null alleles are therefore required to study the role of BRCA1 in breast cancer development.
Here we have generated conditional mouse mutants with somatic deletion of Brca1 and p53 in several epithelial tissues including mammary epithelium. Female mice of this strain showed a high incidence of mammary tumors that mimic many aspects of human BRCA1-mutated basal-like breast cancer.
Results
Somatic Inactivation of p53 Induces Mammary Tumor Formation.
Because BRCA1-mutated breast tumors frequently contain mutations in TP53 (20), we set out to create a mouse mammary tumor model based on simultaneous inactivation of BRCA1 and p53. Modeling mammary tumorigenesis in conventional p53 knockout mice is complicated by the fact that these mice develop lymphomas and sarcomas, rather than epithelial tumors (21–23). Moreover, conventional tumor suppressor gene knockout mice do not mimic sporadic tumor development because the targeted mutations are present in all cells of the animal (24). We therefore generated a conditional mammary tumor model based tissue-specific inactivation of p53. To this end, we crossed conditional p53F mice with K14cre transgenic mice in which Cre recombinase expression is restricted to several epithelial tissues, including skin and mammary gland epithelium (25). The resulting K14cre; p53F/F female mice developed mammary tumors and skin tumors with a median latency (T50) of 288 days (Fig. 1A).
Fig. 1.
Incidence and spectrum of tumors in K14cre female mice carrying conditional Brca1F and p53F alleles. (A) Kaplan–Meier (K–M) survival curve of K14cre;p53F/F female mice (n = 32) showing a median tumor-free survival age (T50) of 288 days. (B) K–M curves of K14cre;p53F/F mice (blue curve) versus K14cre;Brca1F/F female mice (green curve; n = 11; T50 = 595 days; P < 0.0001) and K14cre;Brca1F/F;p53F/F female mice (orange curve; n = 50; T50 = 213 days; P < 0.0001). (C) K–M curve of K14cre;p53F/F mice (blue curve) versus K14cre;Brca1F/+;p53F/F female mice (pink curve; n = 19; T50 = 332 days; P = 0.218). (D) K–M curve of K14cre;Brca1F/F;p53F/+ female mice (red curve; n = 16; T50 = 407 days). Tumor types (mammary, skin, or other) are indicated for each female. Mice were killed when mammary tumors reached a diameter of ≈1 cm or skin tumors grew to a size of ≈0.7 cm. For mice with both skin and mammary tumors, the size of the latter was used as the criterion. (E) Southern blot analysis of tumor DNA to detect Cre-mediated deletion and spontaneous loss of Brca1 (EcoRV + StuI digest, Brca1 exon 14 probe) and p53 (BglII digest, p53 XbaI probe). Tumors (T) and control spleens (C) from the same animal are shown for representative female mice (1–6) of each genotype.
BRCA1 and p53 Loss Collaborate in Mouse Mammary Tumorigenesis.
To study the role of BRCA1 in mammary tumorigenesis, we generated mice carrying conditional Brca1F alleles in which Brca1 exons 5–13 are flanked by loxP sites [supporting information (SI) Fig. 7]. We crossed these animals to K14cre and K14cre; p53F/F mice to produce compound mutant animals with epithelium-specific loss of BRCA1 and/or p53. K14cre; Brca1F/F female mice showed normal ductal and alveolar development and were able to lactate and nurse their litters (data not shown). A cohort of 11 K14cre; Brca1F/F virgin mice was monitored for tumor formation; however, none of the animals developed mammary tumors during an 800-day period (Fig. 1B).
To study the potential synergistic effects of epithelial BRCA1 and p53 inactivation on mammary tumor formation, cohorts of K14cre; Brca1F/F; p53F/F, K14cre; Brca1F/+; p53F/F, and K14cre;Brca1F/F; p53F/+ female mice were generated and monitored for the development of neoplasms. Compared with K14cre; p53F/F animals, K14cre; Brca1F/F; p53F/F mice developed mammary tumors with a significantly reduced T50 of 213 days (Fig. 1B; P < 0.001). Tumor onset and progression in these mice were relatively uniform, with most tumors arising between 140 and 280 days. Compared with K14cre; Brca1F/F; p53F/F female mice, significantly longer median tumor-free survival periods (P < 0.0001) were observed for the K14cre; Brca1F/+; p53F/F and K14cre; Brca1F/F; p53F/+ animals (T50 of 332 days and 407 days, respectively), demonstrating that both BRCA1 and p53 loss of function contribute to tumorigenesis in these mice (Fig. 1 C and D). K14cre; Brca1F/+; p53F/F and K14cre; p53F/F animals developed tumors with comparable latency (Fig. 1C; P = 0.218), showing that BRCA1 is not haploinsufficient for suppression of mammary and skin tumorigenesis in mice. The fraction of female mice that developed mammary tumors was higher in the K14cre; Brca1F/F; p53F/F cohort (40 of 50; 80%) than in the K14cre; p53F/F cohort (20 of 32; 63%), indicating that BRCA1 loss skews toward mammary tumorigenesis. Within each group, mammary and skin tumors developed with a comparable median latency, showing that BRCA1 and p53 loss of function also collaborate in skin tumorigenesis.
High Incidence of Loss of Heterozygosity at p53, but Not at Brca1, in Mammary Tumor Formation.
Collaboration of BRCA1 and p53 inactivation in tumorigenesis was confirmed by Southern blot analysis of mammary tumors from K14cre; Brca1F/F; p53F/F, K14cre; Brca1F/+; p53F/F, and K14cre; Brca1F/F; p53F/+ mice. All tumors (32 of 32) from K14cre; Brca1F/F; p53F/F mice had lost all four conditional Brca1F and p53F alleles (Fig. 1E, mice 1 and 2). Similarly, all tumors (11 of 11) from K14cre; Brca1F/F; p53F/+ animals showed Cre-mediated deletion of all conditional alleles as well as loss of the wild-type p53 allele (Fig. 1E, mice 3 and 4). In contrast, in the K14cre; Brca1F/+; p53F/F mice, all tumors (9 of 9) showed loss of both conditional Trp-53 alleles, but only 80% (7 of 9) of the tumors had lost the conditional Brca1 alleles (Fig. 1E, mouse 6), and 20% (2 of 9) of the tumors showed retention of the conditional Brca1 allele (Fig. 1E, mouse 5). None of these tumors showed loss of the wild-type Brca1 allele. The stochastic loss of the conditional Brca1 allele without concomitant loss of the wild-type allele suggests that BRCA1 has relatively weak tumor suppressor activity compared with p53 or that loss of heterozygosity at Brca1 is more infrequent in mice than in humans.
Histopathologic Features of Mammary Tumors in K14cre;p53F/F and K14cre;Brca1F/F; p53F/F Female Mice.
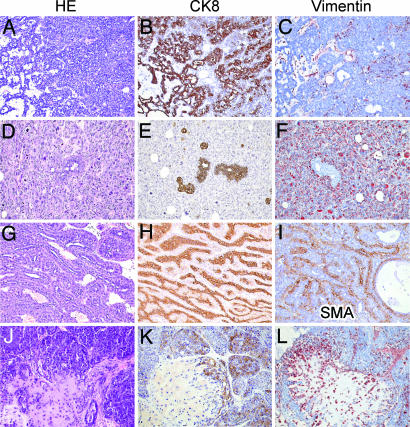
As shown in Table 1 and SI Table 2, the mammary tumors that developed in K14cre; p53F/F female mice were either pure epithelial tumors (8 of 21; 38%), with glandular differentiation or a solid growth pattern without differentiation; or biphasic tumors either poorly differentiated with malignant epithelial and mesenchymal components (10 of 21; 48%) or differentiated with an organoid adenomyoepithelial pattern (3 of 21; 14%). The pure epithelial tumors resembled human invasive ductal carcinoma not otherwise specified (IDC-nos) of intermediate to high grade; all showed expansive growth, high mitotic count, and moderate to high nuclear grade (Fig. 2 A–C). Necrosis was uncommon, and there was no or moderate lymphocytic infiltrate. The cells expressed CK8 and were ER-negative. The poorly differentiated biphasic tumors combined a high-grade CK8-positive epithelial component with a pleomorphic (malignant fibrous histiocytoma-like) CK8-negative and vimentin-positive mesenchymal component (Fig. 2 D–F). These tumors resembled human carcinosarcomas. The differentiated biphasic tumors resembled human adenomyoepitheliomas, showing complex branching glands lined by a double layer of CK8-expressing luminal epithelium and smooth muscle actin-positive myoepithelium (Fig. 2 G–I). Also these tumors had pushing borders, were estrogen receptor (ER)-negative and showed no or moderate lymphocytic infiltrate.
Table 1.
Mammary tumor spectrum and incidence in K14cre;p53F/F and K14cre;Brca1F/F;p53F/F female mice
| Tumor type | Human classification | Incidence (%) |
|
|---|---|---|---|
| K14cre;p53F/F | K14cre;Brca1F/F;p53F/F | ||
| Carcinoma | Intermediate to high-grade IDC-nos | 8/21 (38) | 29/32 (91) |
| Biphasic carcinoma, poorly differentiated | Carcinosarcoma | 10/21 (48) | 1/32 (3) |
| Biphasic carcinoma, well differentiated | Adenomyoepithelioma | 3/21 (14) | 2/32 (6) |
Fig. 2.
Histopathological features of mammary tumors from K14cre;p53F/F and K14cre;Brca1F/F;p53F/F female mice. Microphotographs of representative tumor sections stained with H&E (A, D, G, and J), or with antibodies against CK8 (B, E, H, and K), vimentin (C, F, and L), or smooth muscle actin (SMA) (I). (A–C) Solid carcinoma, resembling high-grade IDC-nos in humans. (D–F) Poorly differentiated biphasic carcinoma, resembling carcinosarcoma in humans. (G–I) Well differentiated biphasic carcinoma, resembling adenomyoepithelioma in humans. (J–L) Carcinoma with chondroid metaplasia.
Compared with K14cre; p53F/F animals, a much larger fraction (29 of 32; 91%) of K14cre; Brca1F/F; p53F/F female mice developed carcinomas that resembled high-grade IDC-nos in humans, with a solid growth pattern, a large CK8-positive and ER-negative cell type with high-grade nuclei, high mitotic count, and with pushing borders (Table 1 and SI Table 3). Few cases showed necrosis, and no or moderate lymphocytic infiltrate was observed. Some Brca1Δ/Δ; p53Δ/Δ carcinomas (2 of 29; 7%) contained areas of chondroid metaplasia, with gradual transitions of solid nests of CK8-positive cells into dispersed CK8-negative, vimentin-positive (myoepithelial) cells surrounded by matrix (Fig. 2 J–L).
BRCA1 Loss Induces Genomic Instability.
Genomic instability is an almost universal characteristic of solid tumors in humans (26), and both BRCA1 and p53 have been implicated in this phenomenon (27, 28). To assess the contribution of each tumor suppressor to genomic instability, we performed array-based comparative genomic hybridization (CGH) analysis on mammary tumors from K14cre; p53F/F and K14cre; Brca1F/F; p53F/F female mice. Although somatic inactivation of p53 alone resulted in an appreciable level of genomic instability, as measured by the extent of DNA copy number aberrations (CNAs), combined inactivation of BRCA1 and p53 caused a significant increase in CNAs (P = 0.00013), indicating that BRCA1 loss of function is an important determinant for induction of genomic instability (Fig. 3).
Fig. 3.
Loss of BRCA1 induces genomic instability. (A and B) Array-CGH profiles of representative mammary tumors from K14cre;p53F/F and K14cre;Brca1F/F;p53F/F mice, respectively. Log2 hybridization ratios are plotted for 2,803 BAC clones, represented on the CGH microarray, at their genomic position. Red dots represent amplifications >0, and green dots represent deletions <0 (Rosetta error model; P < 0.01). (C) Comparison of array-CGH profiles of tumors from K14cre;Brca1F/F;p53F/F (n = 26) and K14cre;p53F/F (n = 27) mice shows that BRCA1 loss results in a significant increase of CNAs. Depicted are mean percentages of BACs with a P < 0.01 per tumor group and per chromosome. Statistical significance of the observed differences in percentage of CNAs between both groups was calculated using a two-sided t test (P = 0.00013).
BRCA1-Deficient Mouse Mammary Tumors Express Basal Epithelial Markers.
To explore differences in gene expression between BRCA1-deficient and BRCA1-proficient mammary tumors, we determined the gene expression profiles of 32 Brca1Δ/Δ; p53Δ/Δ tumors and 21 p53Δ/Δ tumors by using pooled RNA of 12 p53Δ/Δ tumors as a common reference. After combination of the normalized data from dye swap experiments using the Rosetta error model (29), 5,237 genes were selected that showed significant changes in expression across the 53 tumors. Next, a 2D unsupervised hierarchical clustering algorithm was used to group the 53 tumors on the basis of similarity in their gene expression profiles and the 5,237 genes on the basis of similarity in their expression pattern across all 53 tumors (Fig. 4A and SI Fig. 8). This analysis yielded several groups of clustered genes, which contained basal CKs (Krt2–5) and other markers (Trim29, Trp-63, and Idb4) that have been implicated in basal-like breast cancer (9, 30–32). These markers, which are often expressed in human BRCA1-mutated breast cancers (9, 32), were also up-regulated in most, if not all, mouse Brca1Δ/Δ; p53Δ/Δ tumors. Increased expression of p63 and CK5 in Brca1Δ/Δ; p53Δ/Δ tumors, compared with p53Δ/Δ tumors, could be confirmed by immunohistochemistry (Fig. 4 B–G).
Fig. 4.
Mammary tumors from K14cre;Brca1F/F;p53F/F female mice display characteristics of basal-like breast cancers. (A) Unsupervised hierarchical clustering of gene expression profiles from mammary tumors of K14cre;Brca1F/F;p53F/F (n = 32) and K14cre;p53F/F (n = 21) mice. Two-dimensional clustering based on Pearson correlation coefficients revealed three branches of genes that contained several markers for basal cell types. The complete heat map of all 5,237 significant genes is depicted in SI Fig. 8. The dendrogram shows that the majority of the p53Δ/Δ tumors (in blue) were separated from the Brca1Δ/Δ;p53Δ/Δ tumors (in orange). (B–G) Histochemical staining of mammary tumor sections from K14cre;Brca1F/F;p53F/F mice (B–D) and K14cre;p53F/F animals (E–G), with H&E (B and E) or with antibodies against p63 (C and F) and CK5 (D and G).
Supervised Classification of Mouse Mammary Tumors.
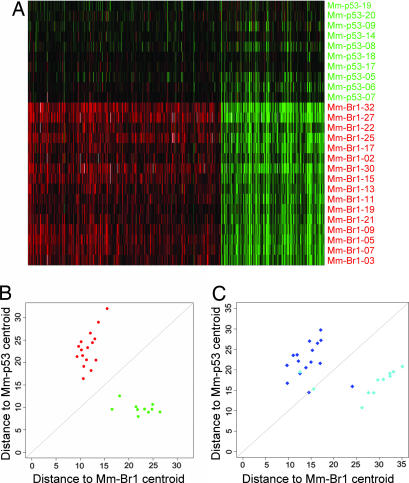
To identify a group of reporter genes that could distinguish mouse Brca1Δ/Δ;p53Δ/Δ tumors from p53Δ/Δ tumors, we performed supervised classification based on significance analysis of microarrays with a training set of 16 Brca1Δ/Δ; p53Δ/Δ carcinomas and 10 p53Δ/Δ carcinomas. We obtained a set of 646 genes (false discovery rate = 0.012) for which expression was either decreased or increased in the Brca1Δ/Δ; p53Δ/Δ tumors, compared with the p53Δ/Δ tumors (Fig. 5A and SI Table 4). A nearest centroid classifier based on the 646 BRCA1 reporter genes was capable of classifying the Brca1Δ/Δ;p53Δ/Δ and p53Δ/Δ tumors from the training set with 100% accuracy (Fig. 5B). Moreover, an 85% (23 of 27) correct classification rate was obtained with the Brca1 classifier for a validation series of 16 Brca1Δ/Δ; p53Δ/Δ and 11 p53Δ/Δ tumors that were not part of the training data set (Fig. 5C). Gene ontology (GO) analysis showed significant overrepresentation of the 646 optimal BRCA1 reporter genes in a number of biological processes, including cell differentiation, cell cycle, and chromatin modification (SI Table 5 and SI Fig. 9).
Fig. 5.
Supervised classification of mouse mammary tumors. (A) Expression data matrix of 646 BRCA1 reporter genes from a training set containing 16 Brca1Δ/Δ;p53Δ/Δ carcinomas (red) and 10 p53Δ/Δ carcinomas (green). Each row represents a tumor, and each column represents a gene. (B) Supervised classification of individual tumors from the training set according to their Euclidean distances to the centroids of mouse Brca1Δ/Δ;p53Δ/Δ mammary tumors and p53Δ/Δ tumors, respectively. (C) Supervised classification of an independent validation series containing 16 Brca1Δ/Δ;p53Δ/Δ tumors (dark blue diamonds) and 11 p53Δ/Δ tumors (light blue diamonds). Most mammary tumors in the validation series (23/27) were correctly classified by the 646 reporter genes.
Cross-Species Comparison by Unsupervised Clustering.
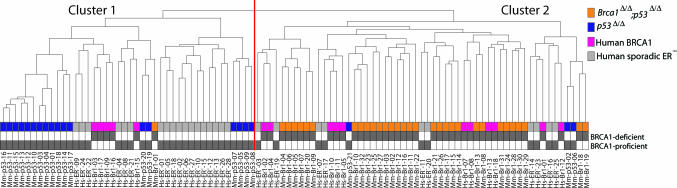
To determine whether the molecular signatures of mouse Brca1Δ/Δ; p53Δ/Δ mammary tumors resembled those of human BRCA1-mutated breast cancers, we examined the relationships among a group of 53 mouse mammary tumors (21 p53Δ/Δ tumors and 32 Brca1Δ/Δ; p53Δ/Δ tumors) and 44 human breast tumors (16 ER-negative BRCA1 tumors and 28 ER-negative sporadic tumors) using unsupervised clustering (Fig. 6A and SI Fig. 10). The first bifurcation of the hierarchical clustering dendrogram identifies two clusters of tumors, which represent nonrandom samplings from the complete population (χ2 test; P = 3.7 × 10−4 and P = 2.5 × 10−5, respectively). Mouse p53Δ/Δ tumors are overrepresented in Cluster 1 (hypergeometric test; P = 6.9 × 10−7), and mouse Brca1Δ/Δ; p53Δ/Δ tumors are overrepresented in Cluster 2 (hypergeometric test; P = 4.2 × 10−11). The human sporadic ER-negative tumors show a significant association with Cluster 1, whereas the human BRCA1 tumors show a significant association with Cluster 2 (χ2 test; P = 0.035). Hence, human ER-negative sporadic breast cancers cluster together with mouse p53Δ/Δ mammary tumors, whereas human BRCA1 tumors cocluster with mouse Brca1Δ/Δ; p53Δ/Δ tumors.
Fig. 6.
Cross-species comparison of mouse and human BRCA1-deficient breast cancers. Unsupervised hierarchical clustering was used to compare gene expression profiles of human breast cancer samples from 16 BRCA1 mutation carriers (pink) and 28 sporadic ER-negative cases (gray), with profiles of 32 mammary tumors from K14cre;Brca1F/F;p53F/F female mice (orange) and 21 tumors from K14cre;p53F/F female mice (blue). Most mouse Brca1Δ/Δ;p53Δ/Δ and human BRCA1-mutated tumors (42 of 48) are interspersed and cluster together in a single branch of the dendrogram. The complete heat map of all 5,410 significant genes is shown in SI Fig. 10.
Cross-Species Comparison by Gene Set Enrichment Analysis (GSEA).
To further explore the similarity between the mouse and human tumors, we used GSEA (33). Based on the expression of 7,127 nonredundant, annotated genes represented on both mouse and human microarray platforms, GSEA was applied to test association between expression of the genes in a gene set and the class labels (i.e., Brca1Δ/Δ; p53Δ/Δ vs. p53Δ/Δ for mouse tumors, and BRCA1-mutated vs. sporadic ER-negative for human tumors). SI Fig. 11 B and C depicts scatter plots of the mouse and human maximum enrichment scores for 980 gene sets of the GO hierarchy (www.geneontology.org) and 424 gene sets of the MSigDb C2 pathways database (www.broad.mit.edu/gsea), respectively. Overall, similar sets of genes exhibit equal levels of association between the gene expression and the class labels for both species, meaning that if a given gene set is predictive for the class label of the human tumors, the same set is also likely to be predictive for the class label of the mouse tumors. In support of this, a significant overlap (24/98) is observed for the top-10% GO gene sets with maximal enrichment scores based on human data and mouse data, respectively (hypergeometric test; P = 7.7 × 10−6). The overlap of the top 10% of the MSigDb gene sets (11 of 42) also is significant (P = 1.1 × 10−3). Several of these overlapping top 10% of the gene sets are associated with processes in which BRCA1 is implicated, such as recombinatorial repair, mitotic recombination, telomere maintenance, X-inactivation, and transcriptional regulation (SI Table 6).
Discussion
To create a mouse model for BRCA1-associated breast cancer, we have exploited two main characteristics of BRCA1-mutated tumors: the frequent occurrence of TP53 mutations and the basal-like phenotype characterized by the expression of basal epithelial markers such as CK5 and CK14. Using Cre-loxP technology, we targeted somatic mutation of both Brca1 and p53 specifically to CK14-expressing (mammary) epithelial cells. The resulting K14cre; Brca1F/F; p53F/F mouse model shows effective cooperation of BRCA1 and p53 in mammary tumorigenesis and a lack of haploinsufficiency for BRCA1 in mammary tumor suppression. The majority of Brca1Δ/Δ; p53Δ/Δ mammary tumors are solid carcinomas that resemble high-grade IDC-nos in humans.
Our histopathological and molecular analyses showed that Brca1Δ/Δ; p53Δ/Δ mammary tumors recapitulate several features of both human BRCA1-mutated hereditary breast cancers and sporadic basal-like breast cancers. Most Brca1Δ/Δ; p53Δ/Δ tumors are highly proliferative, ER-negative carcinomas with a high degree of genomic instability, pushing borders, and expression of basal epithelial markers. The basal-like tumor phenotype might, in part, be explained by the fact that Cre expression in our K14cre transgenic mice is driven by the basal CK14 gene promoter, which is active in mammary stem cells (34, 35).
The availability of mammary tumors from both K14cre; p53F/F and K14cre; Brca1F/F; p53F/F mice allowed us to study the role of BRCA1 loss of function in breast oncogenesis via comparative analysis of matched series of BRCA1-proficient and BRCA1-deficient tumors. Gene expression profiling of Brca1Δ/Δ; p53Δ/Δ mammary tumors and p53Δ/Δ control tumors showed that BRCA1 loss leads to a more undifferentiated tumor phenotype with increased expression of basal epithelial markers, suggesting that BRCA1 might be causally related to the basal-like tumor phenotype. In support of this, BRCA1 has recently been reported to be required for differentiation of mammary epithelial cells (36).
Further support for our mice as a model for human BRCA1-mutated breast cancer came from our cross-species comparisons of microarray expression data from mouse and human breast tumors. Unsupervised clustering of gene expression patterns showed that Brca1Δ/Δ; p53Δ/Δ mouse mammary tumors are most similar to human BRCA1 tumors, whereas p53Δ/Δ mouse tumors are more similar to human sporadic basal-like breast cancers. Similarity between mouse and human BRCA1-deficient tumors is underscored by the fact that Brca1Δ/Δ; p53Δ/Δ mouse mammary tumors mix with human BRCA1-mutated tumors, instead of falling into two separate branches in the dendrogram.
Conventional methods for microarray expression data analysis, such as unsupervised clustering or supervised classification, fail to detect biological processes that are distributed across a gene network and subtle at the level of individual genes. We have therefore used a second analysis method, GSEA, to search for shared relationships between mouse and human BRCA1 tumors with regard to expression patterns of sets of genes with the same biological function or ontology. GSEA of mouse and human BRCA1 tumors showed that, overall, functional gene sets exhibit similar levels of correlation between gene expression and the class labels (i.e., BRCA1-proficient vs. BRCA1-deficient) in both species. Hence, gene sets that are predictive for the class label of the human sample are also likely to be predictive for the class label of the mouse sample. Of note, several of the most predictive gene sets are associated with processes in which BRCA1 is implicated, such as recombinatorial repair and mitotic recombination (5), telomere maintenance (37), X-inactivation (38), and transcriptional regulation (39).
In summary, we have generated a K14cre; Brca1F/F; p53F/F mouse model that develops BRCA1-deficient mammary tumors that closely mimic human BRCA1-mutated breast cancers with basal-like phenotypes. This model may be helpful in predicting responses of human BRCA1-deficient tumors to conventional chemotherapeutics (40), as well as targeted therapeutics such as poly(ADP-ribose) polymerase 1 inhibitors that target cancers with defective homologous recombination repair (41, 42).
Materials and Methods
Mutant Mouse Strains.
Generation of conditional Brca1F mouse mutants is described in SI Materials and Methods. Generation of conditional p53F mutants and K14cre transgenic mice has been described previously (25). All animal experiments were approved by the local ethical review committee.
Southern Blot Analysis and Genotyping.
Isolation of high-molecular weight DNA and Southern blot analysis was carried out as described previously (25). Genotyping of the K14cre and p53F alleles was performed as described (25). Genotyping of Brca1F and Brca1Δ alleles are described in SI Materials and Methods.
Histology and Immunohistochemistry.
Histological and immunohistochemical stainings of tissue sections were performed as described before (43). Primary and secondary antibodies used are listed in SI Materials and Methods.
Array-CGH Analysis.
Array-CGH analysis of mouse mammary tumors was performed by fluorochrome-reversed pairs of two-color hybridizations of 3K mouse BAC microarrays with fluorescently labeled tumor DNA and normal (spleen) DNA from the same animals as described (44). The Rosetta error model was used to calculate weighted averages and statistical confidence levels (29, 44). All values with P values <0.01 were considered to represent significant CNAs. To quantify the difference in genomic instability between p53Δ/Δ and Brca1Δ/Δ; p53Δ/Δ tumors, the total number of significant BACs was summed for all tumors in each tumor group, and a two-sided t test was used to determine statistical significance of the observed differences in values calculated for both groups.
Gene Expression Profiling.
Methods for RNA extraction, RNA amplification, microarray hybridization, and data processing are described in SI Materials and Methods. For unsupervised clustering and supervised classification, the number of gene features was reduced from 18,173 to 5,237 by excluding genes with >10% missing value, as well as genes that showed significant changes in expression (log2 ratio > 1 or < −1) in <10% of the samples.
Unsupervised Clustering and Supervised Classification.
2D unsupervised hierarchical clustering was performed using the Pearson correlation as a distance measure and complete linkage. Significance analysis of microarrays analysis (www.bioconductor.org/packages/1.8/bioc/html/siggenes.html) (45) was used to identify a group of mouse BRCA1 reporter genes, which could separate mouse Brca1Δ/Δ; p53Δ/Δ tumors from p53Δ/Δ tumors in the training set. Supervised classification was performed by calculating the Euclidean distance of each tumor to the centroid of the mouse Brca1Δ/Δ; p53Δ/Δ tumors and p53Δ/Δ tumors, respectively. Tumors used for training and validations are indicated in SI Tables 2 and 3.
GO Analysis of Mouse BRCA1 Reporter Genes.
GO analysis of mouse BRCA1 reporter genes was performed using the Gene Set Analysis Toolkit (www.bioinfo.vanderbilt.edu/webgestalt).
Cross-Species Comparison of Microarray Data.
Methods used for unsupervised hierarchical clustering and GSEA analysis of microarray expression data from human and mouse tumors are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank K. van Veen-Buurman and K. van 't Wout (Netherlands Cancer Institute) for blastocyst injections; Kreatech Biotechnology for providing ULS reagents; M. Mandjes-van Uitert for statistical analysis; the Netherlands Cancer Institute microarray facility, animal facility, and pathology laboratory for providing excellent service; and R. van Amerongen, K. de Visser, and B. Evers for critically reading the manuscript. This work was supported by Netherlands Organization for Scientific Research Grant ZonMw 917.036.347 (to J.J.), Dutch Cancer Society Grant NKI 2002–2635 (to J.J. and A.B.), and Susan G. Komen Breast Cancer Foundation Grant BCTR 0403230 (to J.J.).
Abbreviations
- CGH
comparative genomic hybridization
- CK
cytokeratin
- CNA
copy number aberration
- ER
estrogen receptor
- GO
gene ontology
- GSEA
gene set enrichment analysis
- IDC-nos
invasive ductal carcinoma not otherwise specified
- K–M
Kaplan–Meier
- T50
median latency.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Microarray expression data have been deposited in the ArrayExpress database, www.ebi.ac.uk/arrayexpress (accession no. E-NCMF-6).
This article contains supporting information online at www.pnas.org/cgi/content/full/0702969104/DC1.
References
- 1.Thompson D, Easton DF. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Weber BL. N Engl J Med. 2003;348:2339–2347. doi: 10.1056/NEJMra012284. [DOI] [PubMed] [Google Scholar]
- 3.Scully R, Livingston DM. Nature. 2000;408:429–432. doi: 10.1038/35044000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narod SA, Foulkes WD. Nat Rev Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 5.Venkitaraman AR. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 6.Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, van de Vijver MJ, Farid LM, Venter D, Antoniou A, Storfer-Isser A, et al. J Natl Cancer Inst. 1998;90:1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 7.Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van de Vijver MJ, Parry S, Bishop T, Benitez J, Rivas C, Bignon YJ, et al. Clin Cancer Res. 2005;11:5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 8.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 9.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner N, Tutt A, Ashworth A. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 11.Evers B, Jonkers J. Oncogene. 2006;25:5885–5897. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Genes Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 13.Hakem R, de la Pompa JL, Sirard C, Mo R, Woo M, Hakem A, Wakeham A, Potter J, Reitmair A, Billia F, et al. Cell. 1996;85:1009–1023. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- 14.Gowen LC, Johnson BL, Latour AM, Sulik KK, Koller BH. Nat Genet. 1996;12:191–194. doi: 10.1038/ng0296-191. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig T, Fisher P, Ganesan S, Efstratiadis A. Genes Dev. 2001;15:1188–1193. doi: 10.1101/gad.879201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA, Harris CC, Deng CX. Nat Genet. 2001;28:266–271. doi: 10.1038/90108. [DOI] [PubMed] [Google Scholar]
- 17.Brodie SG, Xu X, Qiao W, Li WM, Cao L, Deng CX. Oncogene. 2001;20:7514–7523. doi: 10.1038/sj.onc.1204929. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 19.Mixon M, Kittrell F, Medina D. Oncogene. 2000;19:5237–5243. doi: 10.1038/sj.onc.1203905. [DOI] [PubMed] [Google Scholar]
- 20.Crook T, Crossland S, Crompton MR, Osin P, Gusterson BA. Lancet. 1997;350:638–639. doi: 10.1016/S0140-6736(05)63327-2. [DOI] [PubMed] [Google Scholar]
- 21.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, Bradley A. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 22.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 23.Purdie CA, Harrison DJ, Peter A, Dobbie L, White S, Howie SE, Salter DM, Bird CC, Wyllie AH, Hooper ML, et al. Oncogene. 1994;9:603–609. [PubMed] [Google Scholar]
- 24.Jonkers J, Berns A. Nat Rev Cancer. 2002;2:251–265. doi: 10.1038/nrc777. [DOI] [PubMed] [Google Scholar]
- 25.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 26.Pihan G, Doxsey SJ. Cancer Cell. 2003;4:89–94. doi: 10.1016/s1535-6108(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 27.Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang XW, Harris CC, Ried T, Deng CX. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 29.Weng L, Dai H, Zhan Y, He Y, Stepaniants SB, Bassett DE. Bioinformatics. 2006;22:1111–1121. doi: 10.1093/bioinformatics/btl045. [DOI] [PubMed] [Google Scholar]
- 30.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A, et al. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro-Silva A, Ramalho LN, Garcia SB, Brandao DF, Chahud F, Zucoloto S. Histopathology. 2005;47:458–466. doi: 10.1111/j.1365-2559.2005.02249.x. [DOI] [PubMed] [Google Scholar]
- 32.Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, Trudel M, Akslen LA. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 35.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 36.Furuta S, Jiang X, Gu B, Cheng E, Chen PL, Lee WH. Proc Natl Acad Sci USA. 2005;102:9176–9181. doi: 10.1073/pnas.0503793102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPherson JP, Hande MP, Poonepalli A, Lemmers B, Zablocki E, Migon E, Shehabeldin A, Porras A, Karaskova J, Vukovic B, et al. Hum Mol Genet. 2006;15:831–838. doi: 10.1093/hmg/ddl002. [DOI] [PubMed] [Google Scholar]
- 38.Ganesan S, Silver DP, Greenberg RA, Avni D, Drapkin R, Miron A, Mok SC, Randrianarison V, Brodie S, Salstrom J, et al. Cell. 2002;111:393–405. doi: 10.1016/s0092-8674(02)01052-8. [DOI] [PubMed] [Google Scholar]
- 39.Welcsh PL, Lee MK, Gonzalez-Hernandez RM, Black DJ, Mahadevappa M, Swisher EM, Warrington JA, King MC. Proc Natl Acad Sci USA. 2002;99:7560–7565. doi: 10.1073/pnas.062181799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rottenberg S, Nygren AOH, Pajic M, van Leeuwen FWB, van der Heijden I, van de Wetering K, Liu X, de Visser KE, Gilhuijs KG, van Tellingen O, et al. Proc Natl Acad Sci USA. 2007;104:12117–12122. doi: 10.1073/pnas.0702955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 42.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 43.Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, et al. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Chung YJ, Jonkers J, Kitson H, Fiegler H, Humphray S, Scott C, Hunt S, Yu Y, Nishijima I, Velds A, et al. Genome Res. 2004;14:188–196. doi: 10.1101/gr.1878804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tusher VG, Tibshirani R, Chu G. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.