Abstract
The malaria parasite Plasmodium falciparum releases the ring-infected erythrocyte surface antigen (RESA) inside the red cell on entry. The protein migrates to the host cell membrane, where it binds to spectrin, but neither the nature of the interaction nor its functional consequences have previously been defined. Here, we identify the binding motifs involved in the interaction and describe a possible function. We have found that spectrin binds to a 108–amino acid fragment (residues 663-770) of RESA, and that this RESA fragment binds to repeat 16 of the β-chain, close to the labile dimer-dimer self-association site. We further show that the RESA fragment stabilizes the spectrin tetramer against dissociation into its constituent dimers, both in situ and in solution. This is accompanied by enhanced resistance of the cell to both mechanical and thermal degradation. Resealed erythrocytes containing RESA663-770 display resistance to invasion by merozoites of P falciparum. We infer that the evolutionary advantage of RESA to the parasite lies in its ability to prevent invasion of cells that are already host to a developing parasite, as well as possibly to guard the cell against thermal damage at the elevated body temperatures prevailing in febrile crises.
Introduction
Invasion by the malaria parasite Plasmodium falciparum brings about extensive changes in the host erythrocyte.1 These include loss of the normal discoid shape, increased rigidity of the membrane, elevated permeability to a wide variety of ionic and other species, and increased adhesiveness, most notably to endothelial surfaces. These effects facilitate survival of the parasite within the host cell and tend to increase the virulence of disease, and indeed P falciparum is the agent of the most severe of all human parasitic diseases, in terms of mortality. It is generally believed that the numerous proteins secreted by the internalized parasite are responsible for the structural changes in the host cell. The erythrocyte is the presumptive target of some 400 P falciparum proteins, of which 225 are virulence proteins and 160 may be involved in erythrocyte remodeling.2–4 To date, 4 proteins have been fairly extensively studied, namely the P falciparum erythrocyte membrane protein 1 (PfEMP1), the knob-associated histidine-rich protein (KAHRP), the mature parasite–infected erythrocyte surface antigen (MESA) and the ring parasite–infected erythrocyte surface antigen (RESA). PfEMP1 is a transmembrane protein encoded by members of a multigene family5 and is exposed at the red cell surface and attached to the membrane skeleton through interactions with spectrin, actin, and KAHRP.6–8 It has been shown that KAHRP binds to repeat 4 of the spectrin α-chain9 and is critically important for both knob formation in infected red cells and the strengthening of the adhesive interactions mediated by PfEMP1.10,11 MESA is a phosphoprotein that has been shown to bind to protein 4.1R, displacing the host protein p55.12–14 This interaction appears to be important for intraerythrocytic growth of the parasite, since the viability of MESA(+) parasites was found to be reduced in 4.1R-deficient erythrocytes.15
RESA (also known as Pf155) is a 155-kDa protein encoded by a 2-exon gene on chromosome 1.16 It contains 2 blocks of repetitive sequence, called the 5′ and 3′ repeats. Between the 2 repeat regions is a segment of 70 residues with similarity to the J domain of E coli and human DnaJ chaperone proteins, suggesting that RESA may have some chaperone-like properties. RESA is synthesized in mature-stage parasites, in which it is stored in organelles known as dense granules.17 Following invasion, it is released into the host cell cytosol, where it is phosphorylated18 and becomes associated with the membrane of the newly invaded cell. RESA remains detectable in the infected erythrocytes until about 18 to 24 hours after invasion, when it gradually disappears as MESA appears.19
Spectrin exists in the cell predominantly as an α2β2 tetramer, which has the form of a long, flexible rod, with a contour length of 200 nm. The protein is characterized by a succession of repeating units (21⅓ in the α-spectrin chain, and 16⅔ in the β-chain), each of about 106 residues, folded into a left-handed, antiparallel triple helical coiled-coil structure.20–22 The 280-kDa α-spectrin and the 246-kDa β-spectrin form antiparallel heterodimers, which in turn self-associate by head-to-head interaction to form the tetramer.23 This involves the binding of a solitary α-helix at the N-terminus of the α-chain to a complementary incomplete repeat, consisting of 2 α-helices at the C-terminus of the β-chain.24 The tetramers can undergo transient dissociation into their constituent dimers, particularly when the cells undergo deformation under shear.25
Spectrin has been identified as the primary attachment site for RESA in the infected erythrocytes,18 but the interaction could not at the time be structurally characterized and its functional implications remained unknown. Here, we show that RESA binds to repeat 16 of β-spectrin (βR16) and that this attachment stabilizes the spectrin tetramer relative to the dimer, both in solution and in the erythrocyte. We further demonstrate that the RESA-induced stabilization of the tetramer is accompanied by a large elevation in mechanical stability of the membrane, by reduced susceptibility to heat-induced vesiculation, and by increased resistance to further malarial invasion of the cell.
Materials and methods
Materials
pMAL vector, myelin basic protein (MBP) resin, monoclonal anti-MBP antibody, T4 ligase, and restriction enzymes were obtained from New England BioLabs (Beverly, MA). Top Pfu polymerase and BL21(DE3) bacteria were from Stratagene (La Jolla, CA); reduced-form glutathione and isopropyl β-d-thiogalactopyranoside (IPTG), from Sigma (St Louis, MO); dextran T40, from Amersham Pharmacia Biotech AB (Uppsala, Sweden); proteinase inhibitor cocktail set II, from Calbiochem (San Diego, CA); sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoresis reagents, from Bio-Rad (Hercules, CA); and GelCode Blue Reagent, from Pierce (Rockford, IL). HRP-conjugated antimouse IgG and HRP-conjugated antirabbit IgG were from Jackson ImmunoResearch Laboratory (West Grove, PA) and Renaissance chemiluminescence detection kit, from Pierce. The CM-5 sensor chip, amino coupling kit, and other reagents for surface plasmon resonance assay were purchased from BIAcore (Piscataway, NJ). YOYO-1 was from Molecular Probe (Carlsbad, CA), and RNAase A from QIAGEN (Valencia, CA). Antispectrin antibody was prepared and characterized in our laboratory. The studies that involve drawing blood from healthy volunteer donors (protocol no. 370) have been approved by the New York Blood Center's institutional review board. The donors provided informed consent in accordance with the Declaration of Helsinki.
Construction of recombinant spectrin, RESA, and KAHPR fragments
Recombinant spectrin fragments and single repeats were subcloned into pGEX-4T-2 vector as described previously.26,27 The RESA cDNA encompassing nucleotide region 196-2311 was cloned from P falciparum genomic DNA and was used as template to amplify the 5 RESA fragments, F1 (65-184), F2 (185-346), F3 (347-505), F4 (505-662), and F5 (663-770). All were subcloned into pMAL-2X vector using BamHI and SalI cloning sites upstream and downstream, respectively. The KAHRP fragment, K2a+b, which has been shown to bind to repeat 4 of α-spectrin,9 was also cloned into pMAL vector to improve its solubility.
Preparation of spectrin and recombinant proteins
Spectrin from erythrocytes was prepared according to Tyler et al.28 To obtain recombinant proteins, the cDNA encoding the desired sequences was transformed into the BL21 bacterial strain. Expression was induced by 0.1 mM IPTG at 4°C overnight. The GST-tagged spectrin fragments were isolated on a glutathione-Sepharose 4B affinity column. The MBP-tagged RESA and KAHRP polypeptides were purified on an amylose resin affinity column. Proteins were dialyzed against binding buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl) for pull-down assays, against HBS-EP buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, and 0.05% [vol/vol] surfactant P20) for surface plasmon resonance assay, or against hypotonic buffer (5 mM Tris, 5 mM KCl, pH 7.4) for resealing. Concentrations of spectrin and recombinant GST-tagged spectrin fragments were determined spectrophotometrically, using extinction coefficients calculated from the tryptophan and tyrosine contents.29 Concentrations of recombinant MBP-tagged RESA and KAHRP fragments were estimated by the Bradford method, using BSA as standard.
Pull-down assays
To examine the binding of full-length spectrin to RESA, MBP-tagged RESA polypeptides were coupled to amylose beads at room temperature for 30 minutes. Beads were pelleted and washed. Spectrin dimers were added to the RESA-conjugated beads in a total volume of 100 μL. The final concentrations of both coupled polypeptide and spectrin were 1 μM. The mixture was incubated for 1 hour at room temperature, pelleted, washed, and eluted with 10% SDS. The pellet was analyzed by SDS-PAGE, and spectrin brought down was detected by Western blotting, using antispectrin antibody. Conversely, to define the binding site of the RESA fragment on spectrin, GST-tagged recombinant spectrin fragments were incubated with beads bearing coupled RESA F5. Bound spectrin was detected by Western blotting with anti-GST antibody. Other binding assays were performed in a similar manner.
Surface plasmon resonance
Surface plasmon resonance assays were performed in a BIAcore 3000 instrument as described previously.9 Spectrin or GST-tagged spectrin repeat βIR16 was covalently coupled to a CM-5 biosensor chip, using an amino coupling kit, and increasing concentrations of RESA F5 fragment were injected over the surface. Binding reactions were carried out in HBS-EP buffer and the regenerating medium was 50 mM NaOH. Sensograms (plots of changes in binding units, RU, as a function of time) were analyzed with the aid of BIAeval 3.0 software (BIAcore). Affinity constants were estimated by curve-fitting, assuming 1:1 stoichiometry.
Enzyme-linked immunosorbent assay (ELISA)
Spectrin was coated on the 96-well plate overnight at 4°C. The plate was washed and blocked with 1% BSA in PBS, containing 0.05% Tween 20, for 1 hour at room temperature. MBP-tagged RESA F5 (0.1 μM) preincubated with various concentrations of βR16 (or αIR4 as control) was added and the reaction was continued for another 30 minutes. The binding of MBP-tagged RESA F5 to spectrin was detected by anti-MBP antibody. The plate was read at 450 nm.
Introduction of polypeptides into erythrocyte ghosts
MBP, MBP-tagged RESA F5, or MBP-tagged K2a+b was introduced into erythrocyte ghosts as previously described25: red cells were isolated from freshly drawn blood by centrifugation and washed with Tris-buffered isotonic saline (0.15 M potassium chloride, 10 mM Tris, pH 7.4). The cells were lysed and washed 3 times with 35 volumes of ice-cold hypotonic buffer (5 mM Tris, 5 mM KCl, pH 7.4). The ghosts were incubated with gentle mixing with the required concentrations of the polypeptides in the cold for 10 minutes; 0.1 volume 1.5 M potassium chloride, 50 mM Tris, pH 7.4, was added to restore isotonicity, and incubation was resumed for another 60 minutes at 37°C to allow resealing.
Measurement of membrane stability
The resealed ghosts were examined in the ektacytometer30 to evaluate the effect of peptide incorporation on the resistance of the cells to mechanical shear. The resealed ghosts were suspended in 40% dextran and subjected to a constant shear stress of 750 dynes cm−2. The laser diffraction signal gives the deformability index (DI), and as the cells fragment, DI decreases. The rate of decrease is a measure of the rate of membrane fragmentation and thus of membrane mechanical stability.
Resealing of erythrocytes
Introduction of MBP, MBP-tagged RESA F5, or MBP-tagged K2a+b into erythrocytes was performed as previously described by the dialysis method.9 Thus 2 mL packed erythrocytes at 50% hematocrit were introduced into a dialysis tube, with or without the desired polypeptides, and dialyzed against 500 mL cold hypotonic buffer (5 mM KPO4, pH 7.4, 20 mM KCl, 1 mM MgCl2, and 1 mM ATP) for 80 minutes in the cold. The erythrocytes were resealed by a further dialysis against 500 mL prewarmed isotonic buffer (5 mM KPO4, 160 mM KCl, 5 mM glucose) for 60 minutes at 37°C.
Analysis of heat-induced membrane vesiculation by flow cytometry
Heat-induced size changes of resealed erythrocytes with and without peptide were assessed in the FACScan flow cytometer (BD Biosciences, San Diego, CA), using the FACSDiva software (BD Biosciences) for data acquisition and analysis. Cell size was estimated from the forward and side scatter intensities, based on at least 105 cells in each condition. Statistical significance of the data were evaluated by the t test.
Invasion of erythrocytes by malaria parasites
The 3D7 clone of P falciparum parasites was maintained by standard routines in RPMI 1640 complete medium.31 Infected erythrocytes were synchronized by the sorbitol method as described before.32 The resulting cells were purified to nearly 100% by Percoll gradient centrifugation.33 The fraction containing mature trophozoite-infected cells was added to resealed erythrocytes to give a starting parasitemia of 2%. The cells were cultured in RPMI 1640 medium for 24 hours and parasitemia was determined by flow cytometry. Invasion was assayed according to Jimenez-Diaz et al.34 After washing twice with PBS, the cells were fixed with 0.025% (vol/vol) glutaraldehyde in PBS, 1 mM EDTA, pH 7.4, at 4°C overnight. They were permeabilized in 0.2 ml 0.25% (vol/vol) Triton X-100 in PBS for 5 minutes, and resuspended in 0.1 mL PBS, containing 1 mg/mL RNAase A. For staining, the cells were incubated with 0.5 μM YOYO-1 in 0.1 mL PBS for 30 minutes at room temperature in the dark. The stained cells were resuspended in 0.5 mL PBS and examined in the flow cytometer; 10 000 cells were counted, and parasitemia was defined as the percentage of YOYO-1–positive cells.
Extraction and analysis of spectrin from resealed ghosts
The extraction and analysis of spectrin were performed as described previously.25 The resealed ghosts were washed 3 times with 30 volumes of ice-cold 0.25 mM sodium phosphate, pH 7.4. Extraction was accomplished by incubation in 0.25 mM sodium phosphate, pH 8.0, at 37°C for 1 hour to yield dimers, or at 4°C overnight for preparation of tetramers. The spectrin was collected by centrifugation at 21 000g and examined by gel electrophoresis in the native state in a Tris-Bicine buffer system, run in the cold. The gels, stained with GelCode Blue, were evaluated by densitometry.
Spectrin tetramer-dimer conversion in solution
Spectrin tetramer (0.1 μM) in the low-ionic-strength extraction medium was incubated at 37°C for 1 hour with or without the RESA F5 fragment. The proportion of dimers and tetramers was determined by gel electrophoresis as described in the previous paragraph.
SDS-PAGE and Western blots
SDS-PAGE was performed in 10% gels. Proteins were transferred to nitrocellulose membrane, incubated for 1 hour in blocking buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 0.5% Tween-20, 5% nonfat dry milk), and probed for 1 hour with the desired primary antibody (polyclonal antispectrin or monoclonal anti-MBP). After several washes, the blot was incubated with anti–mouse or anti–rabbit IgG, coupled to horseradish peroxidase (HRP). After final washes, the blot was developed using the Renaissance chemiluminescence detection kit. All steps were performed at room temperature.
Results
Recombinant protein expression and purification
Recombinant RESA and spectrin fragments are schematically depicted in Figure 1A and 1B, respectively. Recombinant spectrin fragments and single repeats were those described previously.26,27 All MBP-tagged RESA fragments are soluble and their purity was greater than 85%. For reconstitution experiments, we also constructed a soluble MBP-tagged K2a+b fragment of KAHRP, using the His-tagged K2a+b as template.9
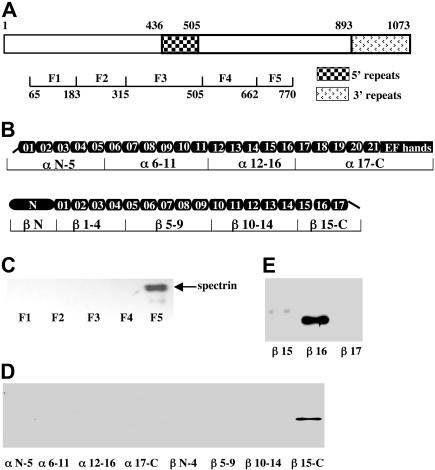
Figure 1.
Identification of spectrin-RESA binding sites. (A) Schematic representation of RESA protein and recombinant fragments. The locations of the 5′ and 3′ repeat regions, and the positions in the sequence of the expressed fragments are indicated. (B) Schematic representation of the spectrin α- and β-chains, showing the domain structure, and locations in the sequence of expressed recombinant fragments. The boundaries of all spectrin fragments and single repeats were defined by SMART annotations. (C) Binding of spectrin dimer to RESA fragments. Spectrin dimer was incubated for 30 minutes at room temperature with each of the MBP-tagged RESA fragments, and binding was assessed by pull-down assay; the bound fragment was detected by blotting with antispectrin antibody after SDS-PAGE. (D) Binding of recombinant spectrin fragments to the RESA F5 fragment. Recombinant GST-tagged spectrin fragments were incubated with RESA F5 and binding was assayed as described for panel C, using anti-GST antibody for detection. (E) Binding of single GST-tagged β-spectrin repeats to RESA F5 fragment. Binding assays were performed as above. Note binding to βR16 only.
Mapping the spectrin binding site in RESA
Earlier deletion experiments suggested that a 48–amino acid sequence of RESA (residues 723-770) contains residues critical for the binding of RESA to spectrin.35 To examine whether this fragment itself possesses spectrin binding activity, we expressed it with an MBP tag and assayed its capacity to bind to spectrin in a pull-down assay. No binding was detected (data not shown), suggesting that residues 723 to 770 may be necessary but insufficient for binding. We therefore prepared a longer RESA fragment, comprising residues 663 to 770 (designated F5), as well as 4 other recombinant MBP-tagged fragments (F1, F2, F3, and F4), spanning residues 65 to 662, to serve as controls (Figure 1A). Binding of spectrin dimer to these constructs was again assessed by pull-down assays. As shown in Figure 1C, under the stated conditions, only F5, comprising residues 663 to 770, was able to pull down spectrin.
Mapping the RESA binding site in spectrin
A similar approach was used to identify the region of spectrin that binds RESA. Nine recombinant GST-tagged spectrin fragments, encompassing the entire α- and β-spectrin chains, were purified and tested for binding to MBP-tagged RESA F5. As shown in Figure 1D, only β15-C showed binding. There was no detectable binding to GST alone. Furthermore, as Figure 1E shows, RESA F5 was found to bind to only 1 of the 3 structural repeats that constitute the β15-C fragment; this was repeat 16 of β-spectrin (βR16). Thus binding of RESA to spectrin is confined to a single one of the 37 repeats of α- and β-spectrin.
Interactions between RESA and spectrin by surface plasmon resonance
The identity of the binding site for RESA in spectrin was confirmed, and the binding was further characterized, by surface plasmon resonance. The spectrin dimer or GST-tagged βR16 was immobilized on the surface of a sensor chip and the course of binding of the RESA F5 fragment was followed. As in the pull-down assay, MPB-tagged RESA F5, but not MBP alone, bound to the immobilized spectrin dimer or to βR16. Concentration dependence of binding of RESA F5 to spectrin dimer and to βR16 yielded dissociation constants of 8.8 × 10−7 M and 5.4 × 10−7 M, respectively. Since the best fit of the data was obtained with 1:1 binding model, we assume that the stoichiometry of the interaction is 1:1.
Inhibition of spectrin binding to RESA F5 by spectrin repeat βR16
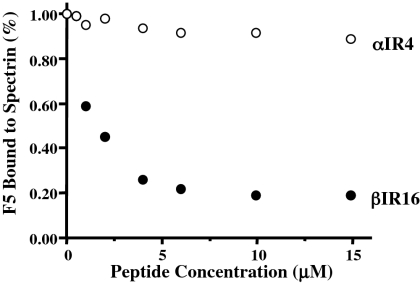
A competitive inhibition assay confirmed the specificity of the interaction between spectrin repeat βR16 and RESA. RESA F5 was incubated with varying concentrations of βR16 or αR4 (repeat 4 of α-spectrin) and added to wells of an ELISA plate containing immobilized spectrin dimer. The binding of RESA F5 to the spectrin was progressively suppressed by increasing concentrations of βR16 (Figure 2). A βR16 concentration of 2 μM inhibited RESA binding by about 50%. By contrast, αR4, a repeat known to bind to KAHRP and to inhibit the binding of intact spectrin to KAHRP,9 did not significantly affect the spectrin-RESA interaction.
Figure 2.
Inhibition of binding of RESA F5 fragment to spectrin dimer by spectrin repeat βR16, assessed by ELISA. RESA F5 was incubated with increasing concentrations of βR16 or αR4 before addition to immobilized spectrin dimer in the ELISA wells. RESA F5 binding to spectrin is inhibited by βR16, but not by αR4.
RESA F5 stabilizes spectrin tetramer
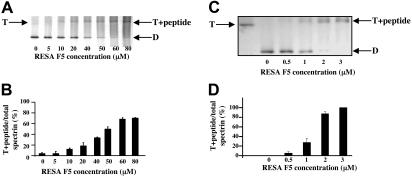
The observation that RESA binds to βR16, which is close to the dimer self-association site and known to play a part in the interaction,24 prompted us to examine whether RESA perturbs the dimer-tetramer equilibrium. We therefore extracted spectrin at 37°C and low ionic strength from ghosts that had been resealed with RESA F5. Whereas the spectrin recovered from control ghosts in these conditions is devoid of tetramer, much of the extract from the ghosts containing RESA F5 was in the form of an F5-tetramer complex (Figure 3A,B). Since the composition of the extract must reflect the amount of F5 bound at each concentration, we can calculate an approximate association constant of binding of F5 to spectrin in situ; this gives a value of approximately 2 × 104 M−1 at 37°C. The conclusion that RESA indeed stabilizes the spectrin tetramer was borne out by its effect on the dimer-tetramer equilibrium in solution. Again, attachment of RESA F5 to spectrin tetramer prevented its conversion to dimers in low-salt solution at 37°C (Figure 3C,D). The fragment acts in solution at a lower concentration than in the intact membrane, as expected from the much greater stability of the spectrin tetramers in the latter state,26 due to the high effective 2-dimensional spectrin concentration in situ. The approximate association constant in solution is 1 × 106 M−1 at 37°C, but this pertains to conditions of very low ionic strength, and is indeed lower by about an order of magnitude than the value delivered in physiological ionic strength and at 20°C by surface plasmon resonance (see “Interactions between RESA and spectrin by surface plasmon resonance”).
Figure 3.
Stabilization of spectrin tetramer by RESA F5 fragment. Panels A and B show the composition of spectrin extracted at 37°C from erythrocyte membranes previously resealed without or with the indicated concentrations of RESA F5. Spectrin components were separated by nondenaturing gel electrophoresis in the cold. The positions of migration of dimer (D), tetramer (T), and the tetramer-RESA-F5 complex (T + peptide) are indicated. Panels C and D show suppression of conversion of spectrin tetramer to dimer by RESA F5 in solution. Spectrin tetramer in the low-ionic-strength extraction medium was incubated with the RESA fragment at the indicated concentrations at 37°C for 1 hour, followed by gel electrophoresis, as before. The best-fit of binding data in situ at 37°C shown in panel B corresponds to an association constant of approximately 2 × 104 M−1, while that of binding data shown in panel D results in an association constant of approximately 1 × 106 M−1. (B,D) Results represent the mean value of triplicate samples.
Effect of RESA F5 fragment on erythrocyte membrane stability
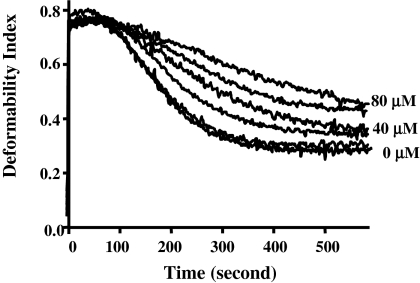
Conversion of any significant proportion of the spectrin tetramers in the red cell membrane to dimers, whether through natural mutations or chemical modification, is accompanied by severe impairment of membrane stability.36 It is also known that a dynamic tetramer-dimer equilibrium, which normally favors the tetramer, prevails in the cell, and that shear-induced deformation of the membrane is accompanied by transient dissociation of the tetramers into dimers.25 Thus one may suppose that if attachment of RESA or its active fragment causes displacement of this equilibrium so as to inhibit dissociation of the tetramers, an enhancement of membrane stability may result. We found this indeed to be the case. Resealed ghosts containing RESA F5 were tested for mechanical stability by shearing in the ektacytometer.30 As Figure 4 shows, the shear resistance (measured by rate of reduction of the light-scattering signal, or deformability index [DI], as the membranes vesiculate) rises with increasing concentration of the RESA fragment. At the highest concentration in the assay (80 μM), we calculate the occupancy of spectrin sites by RESA F5 to be approximately 60%.
Figure 4.
Effect of intracellular RESA F5 fragment on red cell membrane stability. RESA F5 at concentrations of 0, 5, 20, 40, 60, and 80 μM was added to ghosts before resealing. Membrane mechanical stability of resealed ghosts was measured by ektacytometry. The deformability index (DI) measures the deformation of the membranes under constant shear stress. The decay of DI with time is due to progressive breakdown of the cells to vesicles, and thus reflects the extent of shear resistance. The fragmentation profiles for 0, 40, and 80 μM are labeled. It should be noted there is a progression in membrane stability with increasing concentrations of RESA peptide.
Effect of RESA F5 fragment on heat-induced vesiculation of erythrocytes
Da Silva et al37 reported that erythrocytes infected with a RESA(−) laboratory strain of P falciparum (FCR3) were more susceptible to heat-induced fragmentation than those infected with a RESA(+) strain. Another recent study similarly showed that knocking out the RESA gene nullified the capacity of the parasite to induce a large increase in heat resistance of the host cell.38 The inference was drawn that one function of RESA may be to protect the parasitized erythrocyte against destruction during febrile episodes. Our data support this earlier work. Thus, while more than 50% of erythrocytes vesiculated when incubated at 49°C for 30 minutes, this proportion was reduced to 37% in erythrocytes resealed with MBP-tagged RESA F5. Introduction of MBP or MBP-tagged K2a+b into erythrocytes was without effect.
Effect of RESA F5 fragment on malarial invasion
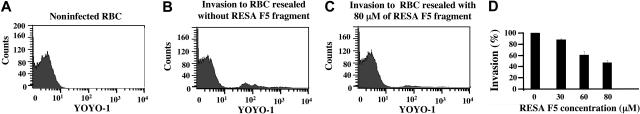
The effect of the RESA fragment on the stability of spectrin tetramers allows us to test whether the spectrin tetramer-dimer equilibrium plays some part in the mechanism of entry of the merozoite. Figure 5 shows that incorporation of MBP-RESA F5 into erythrocyte ghosts causes a very substantial reduction of invasion, while MBP or external MBP-RESA F5 was inert. Thus at an intracellular concentration of 80 μM, corresponding to a calculated occupancy of about 60% of the spectrin sites, invasion is suppressed by some 60%. The profile of invasion against spectrin occupancy suggests complete inhibition at about 80% saturation (but this is beyond what the attainable peptide concentrations allow us to reach). It appears likely, then, that tetramer dissociation is indeed a step that permits or assists entry of the merozoite.
Figure 5.
Effect of membrane-bound RESA F5 fragment on malarial invasion. The proportion of cells infected by P falciparum in culture was assayed by flow cytometry, using YOYO-1 to stain parasite DNA. (A) Uninfected ghosts. (B) Control ghosts (no RESA fragment) exposed to parasites in culture. (C) Ghosts containing 80 μM RESA F5. (D) Dependence of invasion efficiency on intracellular RESA F5 concentration. At the highest concentration of RESA F5 used (80 μM), at which invasion is suppressed by about 60%, the calculated occupancy of spectrin sites in the cell by the peptide is about 60%. Results represent the mean value of triplicate samples.
Discussion
The blood-stage cycle of the malaria parasite is highly complex. It is, for instance, predicted from genomic analyses that the internalized parasite may secrete as many as 400 proteins into the host cell cytosol.2–4 Only a minority of these proteins have had their localization confirmed experimentally or been otherwise characterized, and studies have focused on those proteins that associate with the erythrocyte membrane. Certain of the proteins, such as KAHRP, are known to be involved in knob formation, and thus in adhesion of the parasitized cell to endothelial surfaces, but the function of most others has remained unclear. In a few cases, the receptors in the host cell membrane have been identified and the binding sites closely defined. Thus, MESA binds to 4.1R,13,14 while KAHRP, PfEMP1, and RESA bind to spectrin.7,9,18 We have extended the latter observations by mapping the single binding site for RESA, in the form of its spectrin-binding fragment, F5, to a β-spectrin repeat, close to the dimer-dimer interaction region. The affinity of this interaction in solution appears typical of that recorded for other malaria proteins interacting with the membrane skeleton.9,14,39
The interaction of spectrin with the RESA fragment enhances the stability of the tetrameric relative to the dimeric state of spectrin by a mechanism as-yet unknown. We have shown that elevated resistance of the membrane to shear stress ensues, and confirmed, in agreement with Da Silva et al37 that susceptibility to thermal damage is also reduced. The link between dimer-tetramer balance and mechanical stability is well established and a similar relation between thermal stability may well be a general phenomenon, for it has been noted that the critical temperature at which red cells fragment into vesicles is lowered in hereditary elliptocytes and pyropoikilocytes resulting from spectrin mutations that prevent or weaken the self-association of the dimers.40,41 It is noteworthy that, while red cell membranes can be destabilized through altered spectrin interactions in a variety of ways, the binding of the RESA fragment is, to the best of our knowledge, the first recorded instance of an increase in mechanical membrane stability exerted through a spectrin perturbation.
We are now in a position to draw some inferences concerning the biologic function of RESA. Silva et al38 and Da Silva et al37 have suggested that the elevated thermal stability of the parasitized cells protects them against damage at the body temperatures (sometimes as high as 41°C) in febrile crises. Our results point to an alternative evolutionary function of the RESA protein. Stabilization of spectrin tetramers in situ, increase in shear resistance, and inhibition of merozoite invasion all occur in the same range of added RESA peptide. Reduced invasion is thus presumably linked to suppression of dissociation of the spectrin tetramers into dimers, a conclusion also consistent with earlier observations that antispectrin antibodies, but not their univalent fragments, inside the cell impede entry of the merozoite.42 It has often been noted that in cases of multiple invasion a red cell almost never harbors 2 parasites in different developmental stages. This qualitative observation has been given quantitative expression by Haldar et al (K.H., unpublished observation, January 2007), who exposed a culture rich in trophozoite-containing cells to fresh merozoites and found that only parasite-free cells were invaded. If such secondary invasion were to happen, then presumably the less mature parasite would be killed when the red cell is ruptured by the mature schizont with which it shared a host cell. If this occurred relatively frequently, there would be a deleterious effect on parasite survival. While simultaneous invasion of a cell by 2 or more parasites is commonplace, the selectivity of merozoites for uninvaded cells can be presumed to offer an evolutionary advantage to the parasite, which may be conferred on it by the RESA protein.
The mechanism by which RESA stabilizes the spectrin tetramer, whether through a direct conformational effect or, for instance, a perturbation of the spectrin-ankyrin-band 3 nexus, close to the RESA binding site, will be the subject of further investigations. So also will be the possible effect of RESA on the thermal stability of the spectrin repeat to which it binds. The effect of RESA on the properties of red cells with hereditary defects of spectrin self-association, and other mutations leading to membrane instability, may also be of interest.
Acknowledgments
This work was supported in part by NIH grants DK 26263, DK 32094, and HL78826. R.C. is supported by the Australian National Health and Medical Research Council.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.P., X.G., and S.B. performed research and analyzed the data; K.H. designed experiments; R.C., W.G., and N.M. designed experiments, analyzed the data, and edited the paper; X.A. designed experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiuli An, Red Cell Physiology Laboratory, 310 E 67th St, New York, NY 10021; e-mail: xan@nybloodcenter.org.
References
- 1.Cooke BM, Mohandas N, Coppel RL. The malaria-infected red blood cell: structural and functional changes. Adv Parasitol. 2001;50:1–86. doi: 10.1016/S0065-308X(01)50029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiller NL, Bhattacharjee S, van Ooij C, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 3.Marti M, Baum J, Rug M, Tilley L, Cowman AF. Signal-mediated export of proteins from the malaria parasite to the host erythrocyte. J Cell Biol. 2005;171:587–592. doi: 10.1083/jcb.200508051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sargeant TJ, Marti M, Caler E, et al. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 2006;7:R12. doi: 10.1186/gb-2006-7-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baruch DI, Pasloske BL, Singh HB, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 6.Baruch DI, Gormely JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh SS, Voigt S, Fisher D, et al. Plasmodium falciparum erythrocyte membrane protein 1 is anchored to the actin-spectrin junction and knob-associated histidine-rich protein in the erythrocyte skeleton. Mol Biochem Parasitol. 2000;108:237–247. doi: 10.1016/s0166-6851(00)00227-9. [DOI] [PubMed] [Google Scholar]
- 8.Waller KL, Nunomura W, Cooke BM, Mohandas N, Coppel RL. Mapping the domains of the cytoadherence ligand Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) that bind to the knob-associated histidine-rich protein (KAHRP). Mol Biochem Parasitol. 2002;119:125–129. doi: 10.1016/s0166-6851(01)00395-4. [DOI] [PubMed] [Google Scholar]
- 9.Pei X, An X, Guo X, Tarnawski M, Coppel R, Mohandas N. Structural and functional studies of interaction between Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and erythrocyte spectrin. J Biol Chem. 2005;280:31166–31171. doi: 10.1074/jbc.M505298200. [DOI] [PubMed] [Google Scholar]
- 10.Rug M, Prescott SW, Fernandez KM, Cooke BM, Cowman AF. The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes. Blood. 2006;108:370–378. doi: 10.1182/blood-2005-11-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabb BS, Cooke BM, Reeder JC, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 12.Chishti AH, Maalouf GJ, Marfatia S, et al. Phosphorylation of protein 4.1 in Plasmodium falciparum-infected human red blood cells. Blood. 1994;83:3339–3345. [PubMed] [Google Scholar]
- 13.Lustigman S, Anders RF, Brown GV, Coppel RL. The mature-parasite-infected erythrocyte surface antigen (MESA) of Plasmodium falciparum associates with the erythrocyte membrane skeletal protein, band 4.1. Mol Biochem Parasitol. 1990;38:261–270. doi: 10.1016/0166-6851(90)90029-l. [DOI] [PubMed] [Google Scholar]
- 14.Waller KL, Nunomura W, An X, Cooke BM, Mohandas N, Coppel RL. Mature parasite-infected erythrocyte surface antigen (MESA) of Plasmodium falciparum binds to the 30-kDa domain of protein 4.1 in malaria-infected red blood cells. Blood. 2003;102:1911–1914. doi: 10.1182/blood-2002-11-3513. [DOI] [PubMed] [Google Scholar]
- 15.Magowan C, Coppel RL, Lau AO, Moronne MM, Tchernia G, Mohandas N. Role of the Plasmodium falciparum mature-parasite-infected erythrocyte surface antigen (MESA/PfEMP-2) in malarial infection of erythrocytes. Blood. 1995;86:3196–3204. [PubMed] [Google Scholar]
- 16.Favaloro JM, Coppel RL, Corcoran LM, et al. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986;14:8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Culvenor JG, Day KP, Anders RF. Plasmodium falciparum ring-infected erythrocyte surface antigen is released from merozoite dense granules after erythrocyte invasion. Infect Immun. 1991;59:1183–1187. doi: 10.1128/iai.59.3.1183-1187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley M, Tilley L, Sawyer WH, Anders RF. The ring-infected erythrocyte surface antigen of Plasmodium falciparum associates with spectrin in the erythrocyte membrane. Mol Biochem Parasitol. 1991;46:137–147. doi: 10.1016/0166-6851(91)90207-m. [DOI] [PubMed] [Google Scholar]
- 19.Coppel RL, Lustigman S, Murray L, Anders RF. MESA is a Plasmodium falciparum phosphoprotein associated with the erythrocyte membrane skeleton. Mol Biochem Parasitol. 1988;31:223–231. doi: 10.1016/0166-6851(88)90152-1. [DOI] [PubMed] [Google Scholar]
- 20.Speicher DW, Marchesi VT. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984;311:177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- 21.Yan Y, Winograd E, Viel A, Cronin T, Harrison SC, Branton D. Crystal structure of the repetitive segments of spectrin. Science. 1993;262:2027–2030. doi: 10.1126/science.8266097. [DOI] [PubMed] [Google Scholar]
- 22.Grum VL, Li D, MacDonald RI, Mondragon A. Structures of two repeats of spectrin suggest models of flexibility. Cell. 1999;98:523–535. doi: 10.1016/s0092-8674(00)81980-7. [DOI] [PubMed] [Google Scholar]
- 23.Kotula L, DeSilva TM, Speicher DW, Curtis PJ. Functional characterization of recombinant human red cell alpha-spectrin polypeptides containing the tetramer binding site. J Biol Chem. 1993;268:14788–14793. [PubMed] [Google Scholar]
- 24.Nicolas G, Pedroni S, Fournier C, et al. Spectrin self-association site: characterization and study of beta-spectrin mutations associated with hereditary elliptocytosis. Biochem J. 1998;332(pt 1):81–89. doi: 10.1042/bj3320081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An X, Lecomte MC, Chasis JA, Mohandas N, Gratzer W. Shear-response of the spectrin dimer-tetramer equilibrium in the red blood cell membrane. J Biol Chem. 2002;277:31796–31800. doi: 10.1074/jbc.M204567200. [DOI] [PubMed] [Google Scholar]
- 26.An X, Guo X, Sum H, Morrow J, Gratzer W, Mohandas N. Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry. 2004;43:310–315. doi: 10.1021/bi035653h. [DOI] [PubMed] [Google Scholar]
- 27.An X, Guo X, Zhang X, et al. Conformational stabilities of the structural repeats of erythroid spectrin and their functional implications. J Biol Chem. 2006;281:10527–10532. doi: 10.1074/jbc.M513725200. [DOI] [PubMed] [Google Scholar]
- 28.Tyler JM, Hargreaves WR, Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979;76:5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins SJ. Protein volumes and hydration effects. The calculations of partial specific volumes, neutron scattering matchpoints and 280-nm absorption coefficients for proteins and glycoproteins from amino acid sequences. Eur J Biochem. 1986;157:169–180. doi: 10.1111/j.1432-1033.1986.tb09653.x. [DOI] [PubMed] [Google Scholar]
- 30.Bessis M, Mohandas N, Feo C. Automated ektacytometry: a new method of measuring red cell deformability and red cell indices. Blood Cells. 1980;6:315–327. [PubMed] [Google Scholar]
- 31.Trager W, Jensen JB. Human malaria parasites in continuous culture. 1976. J Parasitol. 2005;91:484–486. doi: 10.1645/0022-3395(2005)091[0484:HMPICC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Aley SB, Sherwood JA, Marsh K, Eidelman O, Howard RJ. Identification of isolate-specific proteins on sorbitol-enriched Plasmodium falciparum infected erythrocytes from Gambian patients. Parasitology. 1986;92(pt 3):511–525. doi: 10.1017/s0031182000065410. [DOI] [PubMed] [Google Scholar]
- 33.Aley SB, Barnwell JW, Daniel W, Howard RJ. Identification of parasite proteins in a membrane preparation enriched for the surface membrane of erythrocytes infected with Plasmodium knowlesi. Mol Biochem Parasitol. 1984;12:69–84. doi: 10.1016/0166-6851(84)90045-8. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez-Diaz MB, Rullas J, Mulet T, et al. Improvement of detection specificity of Plasmodium-infected murine erythrocytes by flow cytometry using autofluorescence and YOYO-1. Cytometry A. 2005;67:27–36. doi: 10.1002/cyto.a.20169. [DOI] [PubMed] [Google Scholar]
- 35.Foley M, Corcoran L, Tilley L, Anders R. Plasmodium falciparum: mapping the membrane-binding domain in the ring-infected erythrocyte surface antigen. Exp Parasitol. 1994;79:340–350. doi: 10.1006/expr.1994.1096. [DOI] [PubMed] [Google Scholar]
- 36.Marchesi SL, Letsinger JT, Speicher DW, et al. Mutant forms of spectrin alpha-subunits in hereditary elliptocytosis. J Clin Invest. 1987;80:191–198. doi: 10.1172/JCI113047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Silva E, Foley M, Dluzewski AR, Murray LJ, Anders RF, Tilley L. The Plasmodium falciparum protein RESA interacts with the erythrocyte cytoskeleton and modifies erythrocyte thermal stability. Mol Biochem Parasitol. 1994;66:59–69. doi: 10.1016/0166-6851(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 38.Silva MD, Cooke BM, Guillotte M, et al. A role for the Plasmodium falciparum RESA protein in resistance against heat shock demonstrated using gene disruption. Mol Microbiol. 2005;56:990–1003. doi: 10.1111/j.1365-2958.2005.04603.x. [DOI] [PubMed] [Google Scholar]
- 39.Bennett BJ, Mohandas N, Coppel RL. Defining the minimal domain of the Plasmodium falciparum protein MESA involved in the interaction with the red cell membrane skeletal protein 4.1. J Biol Chem. 1997;272:15299–15306. doi: 10.1074/jbc.272.24.15299. [DOI] [PubMed] [Google Scholar]
- 40.Zarkowsky HS, Mohandas N, Speaker CB, Shohet SB. A congenital haemolytic anaemia with thermal sensitivity of the erythrocyte membrane. Br J Haematol. 1975;29:537–543. doi: 10.1111/j.1365-2141.1975.tb02740.x. [DOI] [PubMed] [Google Scholar]
- 41.Costa DB, Lozovatsky L, Gallagher PG, Forget BG. A novel splicing mutation of the alpha-spectrin gene in the original hereditary pyropoikilocytosis kindred. Blood. 2005;106:4367–4369. doi: 10.1182/blood-2005-05-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dluzewski AR, Rangachari K, Gratzer WB, Wilson RJ. Inhibition of malarial invasion of red cells by chemical and immunochemical linking of spectrin molecules. Br J Haematol. 1983;55:629–637. doi: 10.1111/j.1365-2141.1983.tb02845.x. [DOI] [PubMed] [Google Scholar]