Abstract
Phosphorylation is a critical step in regulating receptor transport through the endocytic pathway. AAK1 is a serine/threonine kinase that is thought to coordinate the recruitment of AP-2 to receptors containing tyrosine-based internalization motifs by phosphorylating the μ2 subunit. Here we have identified a long form of AAK1 (AAK1L) that contains an extended C-terminus that encodes an additional clathrin-binding domain (CBD2) consisting of multiple low-affinity interaction motifs. Protein interaction studies demonstrate that AAK1L CBD2 directly binds clathrin. However, in vitro kinase assays reveal little difference between AAK1 isoforms in their basal or clathrin-stimulated kinase activity toward the AP-2 μ2 subunit. However, overexpression of AAK1L CBD2 impairs transferrin endocytosis, confirming an endocytic role for AAK1. Surprisingly, CBD2 overexpression or AAK1 depletion by RNA interference significantly impairs transferrin recycling from the early/sorting endosome. These observations suggest that AAK1 functions at multiple steps of the endosomal pathway by regulating transferrin internalization and its rapid recycling back to the plasma membrane from early/sorting endosome.
INTRODUCTION
The adaptor-associated kinase 1 (AAK1) is a member of the Ark1/Prk1 family of serine/threonine kinases that are thought to regulate endocytosis by phosphorylating various endocytic accessory components (Smythe and Ayscough, 2003). AAK1 was originally identified as an interacting partner of the α-adaptin subunit of AP-2 and phosphorylates the μ2 subunit (Conner and Schmid, 2002). The μ2 subunit directly binds tyrosine-based (YxxΦ) internalization motifs found on the cytoplasmic tail of some receptors and mediates cargo recruitment to clathrin-coated vesicles (Ohno et al., 1995). Phosphorylation is thought to be a key regulatory step in cargo recognition as AAK1-mediated μ2 phosphorylation increases the affinity of AP-2 for YxxΦ internalization motif–containing receptors (Ricotta et al., 2002). Although recent observations demonstrate that μ2 phosphorylation is not obligatory for receptor uptake, it is critical for maximizing internalization efficiency (Motley et al., 2006; Olusanya et al., 2001). The resolved crystal structure of the AP-2 “core” has provided an attractive explanation for the molecular basis of the increased receptor affinity of AP-2 after phosphorylation—the YxxΦ binding site within μ2 is buried within the AP-2 core when not phosphorylated (Collins et al., 2002). Thus, μ2 phosphorylation likely results in a conformational change that exposes the YxxΦ binding site and promotes AP-2 recruitment to receptors. Interestingly, AAK1 kinase activity toward μ2 is dramatically stimulated by assembled clathrin (Conner et al., 2003; Jackson et al., 2003), suggesting that AAK1 is specifically activated in clathrin-coated pits to enhance cargo uptake.
The Ark1/Prk1 family is characterized by a high degree of identity within the amino terminal kinase domain of member proteins (Cope et al., 1999). However, little similarity outside the kinase domain exists between family members, arguing that they may have overlapping and divergent functions. For example, Ark1p and Prk1p appear functionally redundant since endocytic defects in yeast are only observed when both genes are disrupted (Cope et al., 1999). Similarly, AAK1 and cyclin G–associated kinase (GAK)/auxilin2 phosphorylate μ2 in vitro and in vivo studies implicate their roles in clathrin-mediated nutrient receptor uptake in mammals (Conner and Schmid, 2002; Zhang et al., 2005). However, GAK/auxilin2 has additional roles in coordinating clathrin-dependent trafficking events at the trans-Golgi (Zhang et al., 2005), the uncoating of clathrin-coated vesicles (Greener et al., 2000), and the trafficking of signaling receptors like epidermal growth factor receptor (Zhang et al., 2004; Lee et al., 2005).
The multiple roles of GAK/auxilin2 in clathrin-mediated transport have, in part, been determined by dissecting the domain structure of the protein. Indeed, its clathrin-binding domain coordinates the recruitment of the Hsc70 chaperone to clathrin-coated vesicles, whereas the DnaJ domain of GAK/auxilin2 stimulates Hsc70-mediated clathrin coat disassembly (Ungewickell et al., 1995; Hannan et al., 1998; Greener et al., 2000; Umeda et al., 2000). Likewise, domain analysis of AAK1 revealed that the α-adaptin–interacting domain is important for AP-2 recruitment and clathrin-mediated stimulation of its kinase activity toward μ2 in vitro (Conner et al., 2003). Interestingly, our initial AAK1 characterization suggested the presence of multiple AAK1 splice variants. Postulating that additional domains within other AAK1 isoforms could provide greater understanding of AAK1 function in clathrin-mediated vesicular transport, we set out to identify additional AAK1 splice forms. Here we identify and characterize a novel AAK1 isoform and find that AAK1, like GAK/auxilin2, functions at multiple steps in clathrin-mediated receptor transport.
MATERIALS AND METHODS
Antibodies
The mAb AP.6 (Affinity Bioreagents, Golden, CO) was used to detect the α-adaptin subunit of AP-2 (Chin et al., 1989), and TD.1 (Covance Research Products, Denver, PA) was used to identify clathrin heavy chain (Nathke et al., 1992). Fluorescein isothiocyanate (FITC)-conjugated EEA1 polyclonal antibodies were obtained from BD Biosciences (San Jose, CA). AAK1L isoform-specific polyclonal antisera (rabbit 273) against a peptide containing the last 17 amino acids of AAK1L (LPNLARSLLLVDQLIDL) conjugated to KLH was commercially produced (Sigma Genosys, St. Louis, MO). Polyclonal antisera against the AAK1 ΔAID region (rabbit 6370) were previously generated and described (Conner and Schmid, 2003b). The mAb E7 that recognizes beta-tubulin was a generous gift from Dr. Jeff Miller (University of Minnesota).
Clathrin and Adaptor Protein Isolation
Clathrin and the adaptor proteins were isolated from bovine brain as previously described (Manfredi and Bazari, 1987).
AAK1L Cloning
The extended 3′ AAK1L fragment was isolated using a nested PCR approach from a random-primed human brain cDNA library (EMD Biosciences, San Diego, CA). The first round of PCR primers included an AAK1 gene–specific primer (5′-ATTCAAGCCCCAGTGAGACAAC-3′) and a vector primer (5′-GGTTATGCTAGTTATTGCTCAGC-3′). The product of this reaction was used for a second round of PCR using two nested gene-specific primers: 5′-AGCCAAAAAGTTCAGACCACTC-3′ and 5′-CTATAGGAGAAGGAAAGGGGT-3′. Full-length AAK1L was then generated by PCR using primers 5′-GAATTCGATGAAGAAGTTTTTCGACTCCCGG-3′ and 5′-GAATTCCAGGTCTATGAGCTGATCCA-3′ both of which incorporated an EcoRI site that was used for subsequent subcloning into baculovirus vectors. PCR products were subcloned into pCR2.1 (Invitrogen, Carlsbad, CA) and sequenced.
Recombinant Protein Production
Full-length AAK1L fusions proteins were generated by subcloning the AAK1L EcoRI fragment from pCR2.1 into the pFastbac1 vector (Invitrogen), which had been previously engineered to contain an in-frame carboxy terminal glutathione S-transferase (GST) or 6HIS affinity tag. The pFastbac1 plasmid was then used to generate baculovirus and subsequently used for protein expression in SF9 cells using the Bac-to-Bac System according to manufacturer protocols (Invitrogen). An AAKL PCR product using primers 5′-GAATTCCAAAGCTGATGTTGCTGTTGAGAGTCTC-3′ and 5′-CTATAGGAGAAGGAAAGGGGT-3′ was subcloned from pCR2.1 into the EcoRI site of pGEX4T-3 (Amersham Pharmacia, Piscataway, NJ) to generate a CBD2-GST fusion construct for bacterial expression in the Rosetta strain (EMD Biosciences).
Adenovirus Production
Adenoviruses encoding an amino terminal myc-tag fused to AAK1L (full-length or CBD2) was generated by subcloning the AAK1L insert from pCR2.1 (SpeI/NotI sites from pCR2.1 used) into the SpeI/NotI site of pADTet7. Short hairpin RNA (shRNA)-encoded adenovirus was constructed by shuttling the H1 histone promoter cassette from pSuper (OligoEngine) into the XhoI site of the pADTet7 plasmid backbone. Hairpin-encoding primers specific for AAK1 were generated (sense: 5′-GATCCCCGTGCTCATTCTGATGGACTTTCAAGAGAAGTCCATC-AGAATGAGCACTTTTTGGAAA-3′; antisense: 5′-AGCTTTTCCAAAAAGTG-CTCATTCTGATGGACTTCTCTTGAAAGTCCATCAGAATGAGCACGGG-3′)and cloned into the BglII/HindIII site of pSuper according to manufacturer protocols before shuttle into pADTet7. The construct was sequence verified before use. The pADTet7 plasmids were then used for adenovirus production as previously described (Damke et al., 1995).
Northern Blot Analysis
Human polyA+ RNA tissue blots (Origene, Rockville, MD) were probed with antisense RNA probes for AAK1 using the Strip-EZ RNA kit (Ambion, Austin, TX) and hybridized according to manufacturer protocols. AAK1 antisense RNA probes were generated using AAK1 cDNA in pCR2.1-containing nucleotides 1777–2466, a region present in both AAK1s and AAK1L isoforms. An AAK1L-specific antisense RNA probe was generated from a pCR2.1 construct containing AAK1L nucleotides 2468–2880. As a control for mRNA loading, a DNA probe was generated using human β-actin cDNA using the DECAprime II kit (Ambion, Austin, TX) according to manufacturer's protocols. All probes were 32P-labeled and hybridized probes were detected by exposing blots to a phophorimaging screen (Amersham Pharmacia) and detected with a Storm scanner (Molecular Dynamics, Sunnyvale, CA).
Protein Interactions
Affinity matrices were generated essentially as described (Conner et al., 2003). Full-length or truncated AAK1 GST fusion proteins immobilized on glutathione-Sepharose beads (Amersham Pharmacia) were incubated for 1 h at room temperature with clathrin triskelia or adaptor proteins from bovine brain. Matrices were then washed with 10 column volumes of phosphate-buffered saline-Tween (PBST) and bound protein was analyzed by SDS-PAGE followed by immunoblot analysis using adaptor protein or clathrin-specific antibodies.
Kinase Assays
Kinase assays were performed as described (Wilde and Brodsky, 1996). Briefly, isolated baculovirus expressed GST fusions of AAK1s or AAK1L with or without adaptor proteins and/or clathrin were mixed in kinase buffer (150 mM KCl, 5 mM MgCl2, 100 μM [γ-32P]ATP) for 40 min at room temperature. The kinase reaction was stopped by the addition of 5× protein sample buffer and boiling for 2 min at 100°C. Proteins were resolved on a 10% polyacrylamide gel, dried, exposed to a phosphoimaging plate, and analyzed using the ImageQuant Software package (Molecular Dynamics). Endogenous kinase activities found in isolated adaptor protein preparations were inactivated by pretreatment with 1 mM 5′-(4-fluorosulfonylbenzoyl) adenosine hydrochloride (FSBA, Sigma) for 60 min at room temperature. Unbound FSBA was removed from protein preparations by gel-filtration using G25 mini-spin columns (Amersham).
Immunolocalizations
For immunofluorescence experiments, HeLa cells were grown on coverslips and then fixed with ice-cold acetone for 10 min followed by methanol extraction. Cells were washed three times with PBST and then incubated with the designated antibodies for 1 h at room temperature. Samples were washed three times with PBST and the appropriate secondary antibodies were added at 1:5000 dilution and incubated for 1 h at room temperature. Samples were visualized by epifluorescence with the appropriate filter set using a Zeiss Axioscop 2 microscope equipped with a Zeiss Axiocam MRm digital camera and 40× and/or 100× objectives. Images were captured using Zeiss Axiovision software release 3.1 (Carl Zeiss, Thornwood, NY).
Single-Round Transferrin Internalization Assay
Stably transformed Tetracycline transactivator (tTA) HeLa cells were cultured in DMEM supplemented with 10% fetal calf serum, 100 U/ml streptomycin/penicillin, and 400 μg/ml G418 as previously described (Sever et al., 2000) and grown to ∼70–80% confluency before use in internalization assays. Cells were detached from 100-mm dishes by the addition of 1.0 ml PBS/5 mM EDTA at room temperature for 5 min and transferred to 1.5-ml microcentrifuge tubes. Cells were gently pelleted by centrifugation at 1000 × g, washed twice with 1.0 ml ice-cold PBS containing 1 mM MgCl2, 1 mM CaCl2, 0.2% bovine serum albumin (BSA), and 5 mM glucose (PBS4+), and resuspended at 2 × 106 cells/ml in PBS4+ containing 5 μg/ml biotinylated transferrin (BXX-Tfn) and incubated 60 min on ice to allow ligand binding to the receptor. Cells were washed twice with ice-cold PBS4+ to remove unbound transferrin, resuspended at 2 × 106 cells/ml in PBS4+, and split into 50-μl aliquots (1 × 105 cells). Samples were then transferred en masse to 37°C for the indicated times. Endocytosis and recycling were stopped by returning samples to ice. Internalized biotinylated transferrin was quantitated by ELISA assay as previously described (Carter et al., 1993).
Recycling Assays
tTA HeLa cells were grown on glass coverslips to ∼70–80% confluency. Before endosomal loading with labeled transferrin, cells were washed with PBS and serum-starved for 30 min at 37°C in DMEM supplemented with 0.5% BSA. Starvation medium was then replaced with prewarmed DMEM/0.5% BSA containing 5 μg/ml Alexa488-labeled transferrin (Invitrogen), and cells were incubated for 60 min at 37°C to allow accumulation of labeled transferrin in the endosomal compartment. After cell loading, a coverslip from each condition was removed, transferred to ice, fixed with 4% formaldehyde, and represented the T = 0 time point. The remaining slides were washed with PBS, the media was replaced with DMEM/0.5% BSA containing 50 μg/ml unlabeled transferrin prewarmed to 37°C and incubated at 37°C to allow recycling from the endosomal compartment back to the plasma membrane. After a 40-min incubation, coverslips were transferred to ice and fixed as described above.
To selectively load the early endosome, cells were processed as before except that the transferrin (biotinylated transferrin) internalization step occurred at 16°C for 60 min as previously described (Strick and Elferink, 2005). Cells were acid-washed and then transferred to 37°C in DMEM/0.5% BSA containing 50 μg/ml unlabeled transferrin for the indicated time. Alternatively, cells were incubated for 10 min at 37°C after the 16°C internalization step to allow accumulation of labeled transferrin in the endocytic recycling compartment. Cells were then acid-washed and returned to 37°C to measure recycling by ELISA (Carter et al., 1993).
RNA Interference
For transfections, silencer Negative control 1 small interfering RNA (siRNA) and AAK1-specific (sense: 5′-GGUGUGCAAGAGAGAAAUCtt-3′; antisense: 5′-GAUUUCUCUCUUGCACACCtg-3′) siRNAs were obtained from Ambion (Austin, TX). tTA HeLa cells (1 × 105) were seeded into each well of a six-well dish containing glass coverslips. For transferrin recycling assays, cells were incubated overnight at 37°C in DMEM containing 10% fetal bovine calf serum. The next morning, cells were washed with PBS, and the medium was replaced with 800 μl of OptiMEM (Invitrogen). siRNA, 100 pmol, in 200 μl OptiMEM was then transfected into cells of each well using 3 μl oligofectamine (Invitrogen), according to the manufacturer's protocols. After a 24-h incubation, 2.0 ml DMEM containing 10% fetal bovine calf serum was added to each well. Seventy-two hours later, cells were assayed for transferrin recycling. In experiments where recycling was quantitated by ELISA, cells were incubated with control or AAK1-specific shRNA-encoded adenovirus for 48 h. After incubation, cells were processed for transferrin recycling as described above.
For rescue experiments, 3 × 105 tTA HeLa cells were plated onto a 35-mm dish containing OptiMEM and incubated 12 h. Cells were then transfected with Lipofectamine 2000 and one of two Steath siRNAs (Invitrogen) targeting the 5′ untranslated region (UTR) of AAK1: Steath siRNA 2: sense 5′-GAGCCGUCUCAAGUUUAAACUUACA-3′; antisense: 5′-UGUAAGUUUAAACUUGAGACGGCUC-3′ (siRNA 2) or Steath siRNA 3: sense 5′GCGCGAUUGACACGCAUAUUCCUAU-3′, antisense 5′-AUAGGAAUAUGCGUGUCAAUCGCGC-3′ (siRNA 3). Cells were incubated for 48 h, split into two 35-mm dishes containing growth medium (DMEM/5% fetal bovine serum [FBS]/400 μg/ml G418) supplemented with 10 ng/ml tetracycline, and then infected with tetracycline-regulatable adenovirus encoding either AAK1 CBD1 as a control or full-length AAK1L. Cells were incubated an additional 24 h before being assayed for transferrin recycling.
RESULTS
AAK1L Is the Predominant Brain Isoform
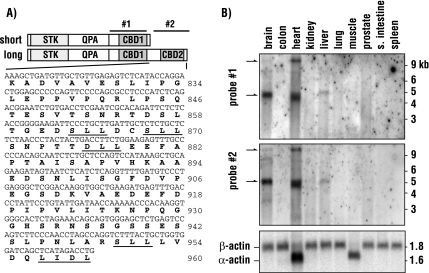
Our previous observations suggested the presence of multiple isoforms of AAK1 (Conner and Schmid, 2002). Immunoblot analysis of bovine brain samples revealed a prominent AAK1 immunoreactive band migrating at a relative molecular weight of ∼140 kDa. However, the full-length KIAA1048 cDNA encoding AAK1 has a predicted molecular weight of 93 kDa, suggesting that the brain form may contain additional functionally relevant domains important for clathrin-dependent intracellular trafficking. To define the predominant AAK1 isoform in brain, we used a PCR-based 3′ RACE strategy to screen a human brain cDNA library (see Materials and Methods). This resulted in the identification of an additional AAK1 isoform that contains an extended 3′ region (Figure 1A). The originally identified AAK1 short form (AAK1s) encodes an 863-amino acid protein, whereas the newly identified long form (AAK1L) encodes 960 amino acids. The two forms are identical through the first 822 amino acids, but possess unique carboxy termini. Interestingly, the carboxy terminal region of AAK1L encodes multiple amino acid motifs that are predicted to bind clathrin with low affinity (Morgan et al., 2000; SLL, DLL, and LIDL; Figure 1A, Wendland et al., 1999), suggesting that AAK1L function is tightly linked to clathrin.
Figure 1.
AAK1L is highly expressed in brain and heart tissue. (A) A schematic representation of the domain structure for the long and short isoforms of human AAK1 indicating the N-terminal serine/threonine kinase domain (STK), the middle domain that is enriched in glutamine, proline, and alanine (QPA), and the two clathrin-binding motif containing domains (CBD1 and CBD2). The regions used to generate antisense RNA probes for Northern blot analysis are indicated as probes 1 and 2. The unique 3′ sequence of AAK1L and its predicted amino acid sequence are indicated below. Underlined amino acid residues indicate predicted clathrin-binding motifs (SLL, DLL, and LIDL). (B) Antisense probes 1 and 2 were used to probe two independent RNA tissue blots as indicated. Blots were also probed for actin to indicate polyA+ mRNA loading. Only one of the two control blots is shown.
To explore potential expression differences between AAK1 isoforms, we performed Northern blot analysis, probing a range of human tissues. An antisense RNA probe targeting the region encoding amino acids 630–822, which is present in both AAK1 isoforms, readily identifies two transcripts (>9 and 5 kb). Brain samples are highly enriched in the 5-kb form, whereas heart tissue reveals both transcripts (Figure 1B). Although not well resolved in the commercial blots, an additional transcript is also present below the 5-kb band. The 5-kb transcript is also detected at lower levels in liver. AAK1 expression in other tissues was below detection despite extended exposure times. Surprisingly, probing a second Northern blot with an antisense RNA probe directed against regions encoding amino acids 823–960 of AAK1L revealed an identical pattern as that observed previously, suggesting that the isoforms expressed in brain, heart, and liver contain the extended carboxy terminal region that encodes additional clathrin-binding motifs. Moreover, these observations demonstrate that AAK1s is likely of very low abundance relative to AAK1L as differences between the two Northern blots were not detected.
To validate the expression of AAK1L, we generated polyclonal antisera against a peptide corresponding to the predicted COOH-terminal 17 amino acids (Figure 1A). Immunoblot analysis of bovine brain lysate (not shown) or protein fractions enriched in clathrin-coated vesicles reveal a single immunoreactive band using AAK1L-specific antisera (Figure 2B). Similarly, a single band is also observed in brain samples using antisera raised against the amino terminal kinase and middle domain of AAK1, demonstrating that AAK1L is indeed the predominant species in brain. On occasion, we observe two closely migrating AAK1 bands in brain samples; however, we believe this represents a proteolytic breakdown product because our Northern blot results indicate a single predominant brain transcript. AAK1L cDNA encodes a predicted 104-kDa protein, a size inconsistent with that observed by immunoblot. However, recombinant baculovirus-expressed full-length AAK1L migrates as predicted (not shown). This anomalous migration in SDS-PAGE is accounted for by the carboxy terminal region of AAK1L (amino acids 823–960). Although this region has a predicted molecular weight of 14.5 kDa, it is acidic (pI 4.1), and bacterially expressed recombinant protein migrates at >40 kDa (not shown).
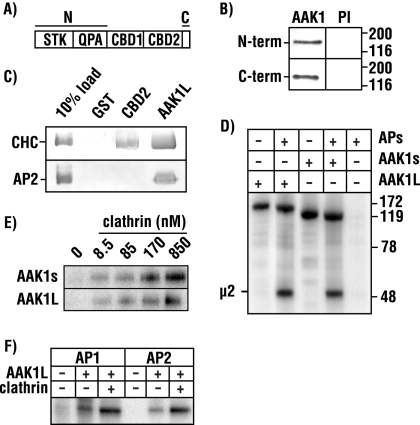
Figure 2.
AAK1 isoforms share similar biochemical properties. (A) The N- and C-terminal regions (see Materials and Methods) used for antibody production are indicated in the AAK1L schematic. (B) Immunoblot analysis of isolated clathrin-coated vesicles probed with N- and C-terminal AAK1 antisera (rabbits 6370 and 273, respectively) reveal a single immunoreactive band. (C) Full-length AAK1L or CBD2 (amino acids 823–960) fused to GST and GST alone were immobilized on glutathione-Sepharose beads and incubated with bovine brain clathrin triskelia or AP2. Clathrin and adaptor protein binding was detected by immunoblot analysis using the monoclonal antibodies TD.1 and AP.6, respectively. TD.1 recognizes the clathrin heavy chain (CHC) and AP.6 identifies the α-adaptin subunit of AP2. The percentage of clathrin or AP2 loaded onto the affinity matrix is indicated. (D) AAK1L- and AAKs-GST fusion proteins were incubated with [γ-32P]ATP in the absence or presence of mixed adaptor proteins. (E) Clathrin stimulation was then tested by supplementing kinase reactions with increasing concentrations of bovine clathrin triskelia, as indicated. (F) Clathrin (850 nM) stimulates AAK1L kinase activity toward μ1 (AP1) and μ2 (AP2). FSBA–inactivated AP1 or AP2 complexes were incubated with [γ-32P]ATP in the presence or absence of AAK1L and clathrin. μ phosphorylation is shown (E and F).
The AAK1L COOH-terminal domain encodes five additional clathrin-binding motifs relative to AAK1s: one DLL motif like that found in the clathrin adaptor/assembly proteins AP180, AP-1, and AP-2, three SLL motifs like those of AP3 and AP4 (Morgan et al., 2000), and an LIDL motif found in the yeast epsins Ent1p and Ent2p (Wendland et al., 1999). The presence of multiple low-affinity clathrin-binding motifs are thought to facilitate the formation of clathrin lattices and/or clathrin cages by cross-linking clathrin triskelia (Morgan et al., 2000). Postulating that this region directly binds clathrin, we tested its interaction with clathrin triskelia in pulldown assays with AAK1 fused to GST. We find that full-length AAK1L or its COOH-terminus (amino acids 823–960) readily interacts with clathrin, whereas GST controls show no clathrin binding (Figure 2C). Not surprisingly, we find that full-length AAK1L, like AAK1s (Conner and Schmid, 2002; Conner et al., 2003), also binds AP-2 (Figure 2C).
AAK1s phosphorylates the μ2 subunit of AP-2, the consequence of which increases the affinity of AP-2 for receptors containing YxxΦ-based internalization motifs (Ricotta et al., 2002). However, AAK1s activity toward μ2 is significantly stimulated by clathrin (Conner et al., 2003; Jackson et al., 2003). Because AAK1L possesses an additional clathrin-binding region, we now refer to as CBD2, we predicted that this region might influence AAK1 kinase activity toward μ2. To test this, we first compared the kinase activity of the long and short forms of AAK1. In vitro kinase assays demonstrate that baculovirus-expressed AAK1L-GST phosphorylates μ2 as effectively as AAK1s-GST (Figure 2D). However, we do not detect any significant differences between isoforms in either autophosphorylation or clathrin-stimulated μ2 phosphorylation (Figure 2, D and E). Similar to AAK1s (Conner and Schmid, 2002), AAK1L phosphorylation of the μ1 subunit of AP-1 is also stimulated by clathrin in vitro (Figure 2F). Together, these observations demonstrate that AAK1s and AAK1L share similar biochemical properties: both interact with clathrin, and they effectively phosphorylate the μ subunits of AP-1 and AP-2 in vitro.
AAK1L CBD2 Overexpression Impairs Transferrin Uptake
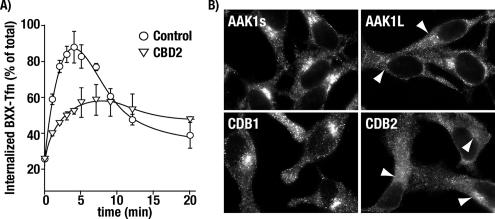
Overexpression of AAK1s disrupts transferrin internalization by functional sequestration of AP-2 (Conner and Schmid, 2003b). Not surprisingly, adenovirus-mediated AAK1L overexpression also sequesters AP-2 and inhibits transferrin uptake (not shown). However, to investigate the functional significance of AAK1L CBD2 in endocytosis, we generated CBD2-encoded adenoviruses for overexpression studies. HeLa cells infected with control tTA-encoded adenovirus rapidly and efficiently internalize surface-bound transferrin within 5 min in single-round uptake assays (Figure 3A). Endocytosed transferrin is then recycled back to the plasma membrane as indicated by a reduction in transferrin from the endosomal compartment. In contrast, AAK1L CBD2 overexpressing cells show a marked reduction in the rate of transferrin uptake (Figure 3A). Similarly, transferrin recycling in these cells also appears impaired relative to the control cells because we see little loss of transferrin once internalized. These observations confirm an endocytic role for AAK1 and suggest that AAK1L may function at multiple steps in the endosomal pathway.
Figure 3.
AAK1L CBD2 overexpression impairs transferrin internalization and disrupts perinuclear clathrin localization. Tetracycline transactivator (tTA) HeLa cells were infected with control adenovirus encoding the (tTA, control) or AAK1L CBD2 for 24 h. Cells were incubated at 4°C for 1 h with biotinylated transferrin (BXX-Tfn) to allow binding to the transferrin receptor, washed, and transferred to 37°C to promote a single round of transferrin internalization (see Materials and Methods). Error bars, the SD of three independent experiments. (B) tTA HeLa cells were infected with the indicated control and AAK1-encoded adenoviruses. Twenty-four hours after infection, cells were fixed, permeabilized, and processed for clathrin immunolocalization. Clathrin was detected by epifluorescence using the mAb TD.1 and an appropriate fluorochrome-tagged secondary antibody. Cells were visualized using a Zeiss Axioscop 2 with attached Axiocam MRm.
Perinuclear Clathrin Localization Is Disrupted after AAK1L CBD2 Overexpression
Because AAK1L CBD2 directly binds clathrin triskelia in vitro (Figure 2C), we reasoned that the transferrin-trafficking defects observed after CBD2 overexpression might result from alterations in clathrin-coated pit formation. To address this possibility, the distribution of clathrin was investigated in AAK1-overexpressing HeLa cells by immunolocalization. Consistent with our previous findings (Conner and Schmid, 2003b), adenovirus-mediated overexpression of full-length AAK1s does not impact clathrin-coated pit formation at the plasma membrane or clathrin recruitment to perinuclear membranes (i.e., trans-Golgi network and endosomes, Figure 3B). Likewise, clathrin recruitment to the plasma membrane after AAK1L overexpression appeared normal; however, a reduction in perinuclear clathrin immunostaining was observed in some cells relative to controls. In contrast, clathrin became somewhat diffuse, and recruitment to perinuclear membranes was markedly reduced in AAK1L CBD2 overexpressing HeLa cells (Figure 3B). This result was specific for CBD2 as overexpression of the first clathrin-binding region of AAK1 (CBD1, a region that overlaps with the α-adaptin interacting domain; Conner and Schmid, 2002) did not impact clathrin localization after overexpression. These observations argue that the observed internalization defect resulting from CBD2 overexpression does not result from gross alterations in clathrin localization at the plasma membrane. Thus we suspect that the endocytic defect results from CBD2 competition with other endocytic components for access to clathrin. However, we cannot rule out the possibility that CBD2, like that which has been observed for the clathrin-binding domain of AP180/CALM (Tebar et al., 1999), more effectively sequesters clathrin from perinuclear membranes.
Because we previously found that full-length AAK1s overexpression functionally sequesters AP-2 and blocks transferrin endoctyosis (Conner and Schmid, 2003a), we speculated that CBD2 overexpression might cause a similar AP-2 redistribution. Although overexpression of full-length AAK1L strongly disrupts AP-2 recruitment to clathrin-coated pits (Supplementary Figure S1), AP-2 localization into discrete punctate structures at the plasma membrane was unaffected by CBD2 overexpression (Supplementary Figure S1). This is consistent with our inability to detect a direct interaction between CBD2 and AP-2 in vitro (Figure 2C). Moreover, in vivo labeling experiments fail to reveal alterations in μ2 phosphorylation after CBD2 overexpression (not shown). Therefore, we conclude that the mechanism by which CBD2 inhibits transferrin uptake is AP-2 independent and that AAK1 interactions with other endocytic components are important for its function.
AAK1 Functions in Transferrin Recycling from the Early/Sorting Endosome
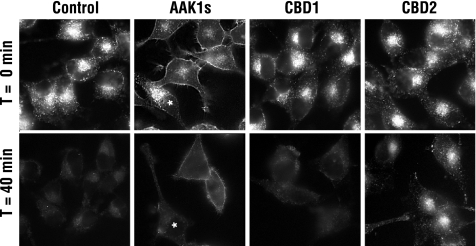
Results from single-round internalization assays (Figure 3A) and defects in clathrin localization to perinuclear endosomal membranes (Figure 3B) suggest that CBD2 overexpression impairs transferrin recycling. To more rigorously test this possibility, HeLa cells grown on coverslips were infected with control or AAK1-encoded adenoviruses and allowed to internalize Alexa488-labeled transferrin for 1 h at 37°C to saturate the endosomal compartment and overcome the AAK1-mediated endocytic block. Cells were then chased with excess unlabeled transferrin at 37°C. After a 40-min chase, control infected cells efficiently recycled transferrin from the endosomal compartment as indicated by a loss of intracellular Alexa488-transferrin (Figure 4). Likewise, AAK1s expressing cells show no significant alteration in transferrin recycling from the endosome, although as a result of the strong endocytic block, the amount of internalized transferrin is significantly less and transferrin accumulates at the plasma membrane. In contrast, CBD2 overexpressing cells show a notable decrease in transferrin recycling, as evidenced by the Alexa488-transferrin that remains in the endosome after the 40-min chase (Figure 4). Because formation of recycling vesicles was recently shown to require clathrin (Pagano et al., 2004), we postulated that overexpression of a clathrin-binding domain might nonspecifically contribute to a recycling defect. To test this possibility, we overexpressed AAK1 CBD1. However, cells overexpressing CBD1 internalize (Conner and Schmid, 2003b) and recycle transferrin at rates indistinguishable from control cells (Figure 4). Thus we conclude that the recycling defects are specific to CBD2 and do not result from nonspecific competition with clathrin.
Figure 4.
AAK1L CBD2 overexpression disrupts transferrin recycling. tTA HeLa cells were infected with tTA control and AAK1s-, CBD1-, or CBD2-encoded adenovirus for 24 h as indicated. Cells were then preincubated with 5 μg/ml Alexa488-labeled transferrin for 1 h at 37°C to saturate the endosomal compartment. Cells were washed at 4°C and returned to 37°C for 40 min to allow Alexa488-transferrin recycling. Cells were then fixed and immediately visualized by epifluorescence using a Zeiss Axioscop 2 with attached Axiocam MRm. Nonoverexpressing cells are indicated with an asterisk.
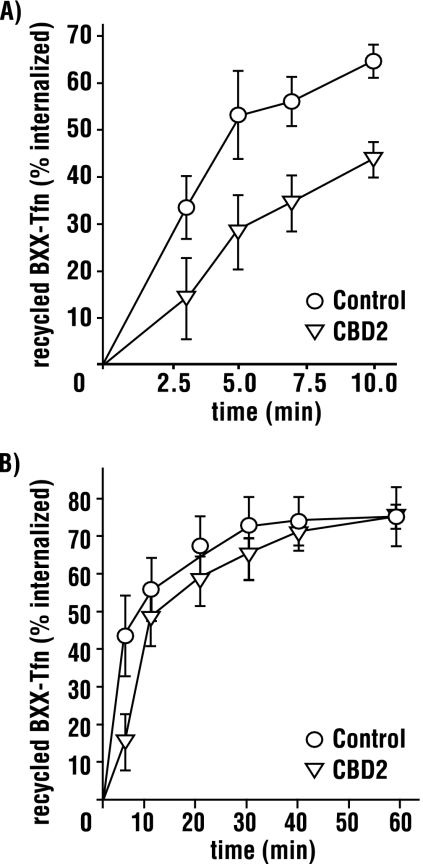
Transferrin recycling occurs via two routes: 1) a rapid pathway directly from the early/sorting endosome (SE) and 2) a slower route through the endocytic recycling compartment (ERC, Maxfield and McGraw, 2004). Although our recycling experiments demonstrate a role for AAK1, they do not differentiate between these two recycling pathways. At 16°C internalized transferrin selectively accumulates in the SE, its recycling back to the plasma membrane is prevented, and transport to the endocytic recycling compartment is blocked (Sipe et al., 1991; Ren et al., 1998; Strick and Elferink, 2005). To determine if AAK1L CBD2 impacts rapid recycling, we repeated recycling experiments as before except that cells were pulsed with biotinylated transferrin at 16°C for 1 h to accumulate labeled transferrin in the SE. After internalization, cells were acid-washed to remove surface-bound transferrin and chased with excess unlabeled transferrin at 37°C. The extent of receptor recycling was then quantitated by ELISA (see Materials and Methods). In cells overexpressing CBD2, we observe a decreased transferrin recycling rate from the SE relative to controls (Figure 5A), demonstrating that CBD2 impairs rapid recycling.
Figure 5.
Rapid recycling from early endosomes is impaired by CBD2 overexpression. tTA HeLa cells were infected with control or AAK1L CBD2 encoded adenovirus for 24 h. Infected cells were incubated for 1 h at 16°C with 5 μg/ml biotinylated transferrin (BXX-Tfn) and acid washed to remove uninternalized ligand. Early endosome recycling was then scored at the indicated time points after transfer to 37°C (A). Alternatively, cells were incubated at 37°C for 10 min to allow Tfn transport to the endosome recycling compartment, acid washed again, and then scored for recycling (B). Error bars, ±SD of 3–4 independent experiments.
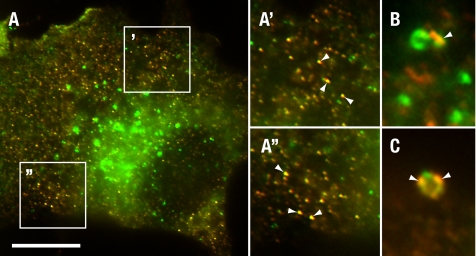
To exclude the possibility that AAK1L CBD2 overexpression also disrupts slower transport from the ERC, HeLa cells were pulsed with biotinylated transferrin for 1 h at 16°C as before. Cells were then acid-washed and chased with excess unlabeled transferrin at 37°C for 10 min to promote recycling from the SE and transport to the ERC as described (Strick and Elferink, 2005). Cells were then transferred to 4°C and acid-washed to remove cell surface transferrin recycled from the SE. Cells were returned to 37°C and chased with excess unlabeled transferrin to measure transport from the ERC. At 5 min we observe a reduction in transferrin recycled from CBD2-overexpressing cells relative to controls (Figure 5B). However, at later time points, little difference was observed. The block at early time points likely reflects the impaired recycling that we observed from the SE and an incomplete transport of labeled transferrin from the SE to the ERC under our experimental conditions. However, because we failed to observe a significant difference in recycled transferrin at later time points, we conclude that CBD2 overexpression does not impair receptor transport from the ERC. Consistent with a role for AAK1 in recycling from the early/sorting endosome, we also found that AAK1 colocalizes with the early endosome antigen EEA1 (Christoforidis et al., 1999) on small peripheral endosomes in HeLa cells (Figure 6A). Moreover, we observed partial overlap with larger EEA1 positive endosomes in some, but not all cells (Figure 6, B and C).
Figure 6.
AAK1 colocalizes with the early endosome. tTA HeLa cells were fixed and stained for AAK1 (red) and early endosome antigen 1 (EEA1, green). AAK1 colocalizes (yellow, arrowheads) with EEA1 at small peripheral endosomes (A, and boxed regions magnified in A′ and A″) and perinuclear endosomes (C and D). AAK1 was visualized with polyclonal antisera raised against the N-terminal domain (rabbit 6370) and EEA1 was identified with affinity-purified, FTIC-conjugated antibodies (BD Biosciences). Scale bar, (A) 10 μm; (A′, A″, C, and D′) 5 μm.
AAK1 Depletion Blocks Transferrin Receptor Recycling
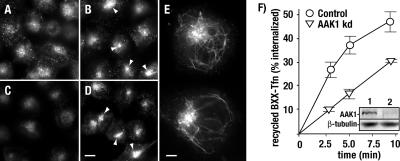
As an alternative strategy to overexpression of AAK1 dominant-negative constructs, we also investigated the impact of RNA interference–mediated AAK1 knockdown on transferrin recycling. Consistent with our previous observations, cells treated with control or AAK1-specific siRNAs efficiently endocytose FITC-labeled transferrin, as evidenced by its intracellular accumulation during a 10-min incubation at 37°C (Figure 7; Conner and Schmid, 2003b). However, cells treated with AAK1 siRNAs accumulate transferrin in a perinuclear compartment to a greater extend than that of controls (Figure 7B). This result is in agreement with published reports (Pelkmans et al., 2005) and suggests a recycling defect. To directly assess the consequence of AAK1 knockdown on transferrin recycling, cells were then washed to remove uninternalized FITC-transferrin and chased with unlabeled transferrin. After a 20-min incubation at 37°C, we find that control cells efficiently recycle transferrin from the endosome (Figure 7C). In contrast, transferrin recycling is impaired in AAK1-siRNA–treated cells where transferrin is found predominantly in the perinuclear endosome (Figure 7D). In addition, we also observe transferrin accumulation in tubular endosomes near the cell cortex (Figure 7E). Consistent with a role in recycling from the sorting/early endosome, AAK1-depleted cells also showed a reduced transferrin recycling rate at early time points by ELISA assay (Figure 7F).
Figure 7.
AAK1 depletion by RNA interference blocks transferrin recycling. HeLa cells were transfected with control (A and C) or AAK1-specific siRNA (B, D, and E). After 72 h, cells were pulsed for 10 min at 37°C with 5 μg/ml FITC-labeled transferrin (A and B), washed, and chased for 20 min with 25 μg/ml unlabeled transferrin (C–E). The recycling block is evident by FITC-transferrin accumulation in perinuclear endosomes (arrowheads) and the tubules of the early/sorting endosome (E). Recycling was quantitated by ELISA at the indicated time points after shRNA-mediated AAK1 depletion (see Materials and Methods). The immunoblot (inset) indicates AAK1 depletion in knockdown cells (kDa, lane 2) relative to the control cells (lane 1). Polyclonal antisera were used to identify AAK1 (rabbit 6370). As a loading control blots were also probed with the mAb against beta tubulin (clone E7). Scale bar, (A–D) 10 μm, (E) 5 μm. Error bars, ±SD of three independent experiments.
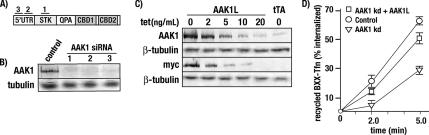
To confirm the specificity of this observation, we next tested the ability of recombinant AAK1L to rescue the transferrin receptor recycling defect after endogenous AAK1 depletion. For these experiments, AAK1 was depleted with siRNAs that targeted the 5′UTR (Figure 8). Cells were then infected with siRNA-resistant AAK1L-encoded adenovirus, which lacked the 5′UTR. Recombinant AAK1L expression was under the control of the tetracycline-regulatable promoter, which allowed us to tightly control expression levels (Figure 8C) and avoid AAK1L-mediated inhibition of transferrin uptake after overexpression. Consistent with a recycling role, we find that recombinant AAK1L expression significantly rescues the rate of transferrin receptor recycling in cells depleted of endogenous AAK1 relative to controls (Figure 8D). From this observation we conclude that the reduced transferrin recycling rates observed after AAK1 siRNA-treatment are specific for AAK1 and that AAK1 plays an critical role in receptor transport from the endosomal compartment back to the plasma membrane.
Figure 8.
Transferrin recycling defects after siRNA-mediated AAK1 depletion are rescued by recombinant AAK1L. (A) Schematic diagram illustrating siRNA-targeted regions of AAK1L. (B) tTA HeLa cells were transfected with control siRNAs or the indicated AAK1 siRNAs (1–3), incubated for 72 h and analyzed for AAK1 knockdown by immunoblot analysis. (C) tTA HeLa cells were infected for 24 h with control (tTA) or tetracycline-regulatable adenovirus encoding myc-tagged AAK1L in the presence of increasing tetracycline concentrations as indicated. Samples were then split in two and analyzed for endogenous and recombinant AAK1 expression using polyclonal AAK1 antisera (rabbit 6370, B and C), or recombinant myc-tagged AAK1 expression with the mAb 9E10. Blots were also probed for β-tubulin (E7) to ensure equal loading. (D) tTA HeLa cells were transfected with control or AAK1 siRNA 2, incubated 48 h, and then left untreated (AAK1 kDa) or infected with adenovirus encoding either AAK1 CBD1 (control) or full-length AAK1L (AAK1 kDa + AAKL) in the presence of 10 ng/ml tetracycline. After an additional 24-h incubation, cells were analyzed for transferrin recycling as described in Figure 7.
DISCUSSION
AAK1 and Receptor Internalization
AAK1-mediated phosphorylation of the AP-2 μ2 subunit is thought to coordinate endocytosis by regulating adaptor recruitment to receptors containing tyrosine-based internalization motifs (Conner and Schmid, 2002; Ricotta et al., 2002). Moreover, clathrin dramatically stimulates AAK1 kinase activity toward μ2 (Conner et al., 2003), an event that promotes receptor incorporation into clathrin-coated pits (Jackson et al., 2003). These observations argue that AAK1 interaction with clathrin is critical to governing clathrin-dependent vesicular transport. Consistent with this, we have identified a long form of AAK1 that contains a second carboxy terminal clathrin-binding domain. Northern blot analysis reveals multiple AAK1 isoforms, all of which encode at least part of CBD2. Surprisingly, the previously described AAK1 short form (KIAA1048) was not observed by Northern blot in brain tissue despite the fact that KIAA1048 was isolated from brain (Kikuno et al., 1999). Our immunoblot studies clearly indicate the AAK1L is the predominant isoform in brain, suggesting that AAK1s represents a minor population.
In vitro binding studies indicate that AAK1L interacts with clathrin through multiple domains, adding support to the idea that AAK1 function is tightly linked to clathrin. Although clathrin interactions with CBD1 are important for stimulating AAK1 kinase activity toward μ2 in vitro (Conner et al., 2003), overexpression of this region does not impact clathrin-mediated transferrin uptake or μ2 phosphorylation (Conner and Schmid, 2003b). In contrast, CBD2 overexpression significantly impairs the rate of transferrin uptake, suggesting that AAK1L has an AP2-independent role in endocytosis, possibly to coordinate clathrin function.
What is the significance of AAK1L-clathrin interactions mediated by CBD2? In vitro and in vivo phosphorylation data argue that CBD2 does not play a role in regulating AAK1L kinase activity. Alternatively, the presence of multiple low-affinity clathrin-binding sites within CBD2 suggests that AAK1L might facilitate clathrin assembly or membrane recruitment. A clathrin assembly role seems unlikely, however, because we were unable to detect a significant impact of CBD2 on clathrin cage formation using well-established in vitro assembly assays (unpublished observations; Engqvist-Goldstein et al., 2001; Legendre-Guillemin et al., 2005). Furthermore, clathrin localization to the plasma membrane is not grossly altered after CBD2 overexpression or by siRNA-mediated AAK1 knockdown (not shown). Thus we speculate that AAK1L might regulate clathrin interactions with adaptor proteins or other accessory factors important for endocytosis. The small clathrin-binding motifs within AAK1L CBD2 are known to bind the terminal domain of clathrin heavy chains (Morgan et al., 2000; ter Haar et al., 2000), a region essential for interactions with AP-2 and AP180/CALM. Therefore, saturation of the terminal domain after CDB2 overexpression might limit access of these adaptors to clathrin, thereby inhibiting clathrin function and endocytosis.
siRNA-mediated depletion suggests that either AAK1 is not essential for transferrin endocytosis or that it is functionally redundant with other kinases like GAK/auxilin2 (Lee et al., 2005; Zhang et al., 2005). Therefore, we postulate that AAK1 plays a nonessential regulatory role in coordinating AP2 function to maximize receptor internalization efficiency. Consistent with this, Motley et al. (2006) recently discovered that a nonphosphorylatable form of μ2 only partially rescues transferrin internalization when endogenous μ2 is depleted by siRNA. This clearly demonstrates that μ2 phosphorylation, although not required, is important for efficient receptor uptake.
Transferrin Recycling from the Sorting Endosome
In addition to a role for AAK1L in clathrin-mediated transferrin uptake, AAK1L CBD2 overexpression and siRNA-mediated AAK1 depletion experiments indicate that AAK1 also functions in receptor recycling from the SE. Current models predict that transferrin recycling from the endosome is largely dependent on organelle geometry rather than on specific receptor sorting motifs (Maxfield and McGraw, 2004). For example, the pinching off of long endosome tubules with a large surface area-to-volume ratio could preferentially sort membrane-bound receptors back to the plasma membrane. Consistent with this, the kinetics of transferrin receptor recycling, independent of its cytoplasmic domain, are identical to that of a bulk membrane marker (Johnson et al., 1993; Mayor et al., 1993). However, when the endosome pH is altered by treatment with bafilomycin, a vacuolar proton pump inhibitor, efficient transferrin recycling then requires an intact cytoplasmic tail (Johnson et al., 1993). This observation argues for multiple recycling pathways, one of which is dependent on cytosolic factors for efficient receptor transport. Indeed, transferrin recycling is partially dependent on clathrin (Wettey et al., 2002) and tubular endosomes have transferrin-containing clathrin-coated buds (Stoorvogel et al., 1996). Moreover, transferrin recycling is partially dynamin-dependent (van Dam and Stoorvogel, 2002). The requirement for cytosolic factors in receptor recycling is consistent with our observations where clathrin displacement from endosomal membranes after CBD2 overexpression or AAK1 depletion results in a block in receptor recycling.
What is the role of AAK1 in receptor recycling? It is possible that AAK1 coordinates receptor sorting at the endosome by regulating adaptor cargo recruitment, similar to that proposed for AAK1 function in endocytosis (Conner and Schmid, 2002; Ricotta et al., 2002; Jackson et al., 2003). An obvious AAK1 target candidate is the adaptor complex AP-1. Consistent with this notion, in vitro kinase assays demonstrate that AAK1L can phosphorylate the μ1 subunit of AP-1 as efficiently as AAK1s. AP-1 binding to receptor sorting signals, similar to AP-2, is regulated by phosphorylation (Ghosh and Kornfeld, 2003). Previous studies in polarized Madin-Darby canine kidney cells indicate that AP-1 is responsible for basolateral sorting of the transferrin receptor (Futter et al., 1998). However, AP-1 association with peripheral endosomes in nonpolarized cells (Waguri et al., 2003; Keyel et al., 2004), and its requirement for the formation of endosomal recycling vesicles in vitro (Pagano et al., 2004) suggests a more general role in receptor recycling. Collectively, our observations when combined with those of others lead us to speculate that similar receptor-sorting mechanisms are used for clathrin-dependent transport from the endosome and the plasma membrane. Thus, future studies will focus on resolving the molecular details of AAK1L function in clathrin-dependent transport and its potential regulation of adaptor proteins like AP1.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge members of the Conner lab for helpful discussions. S.D.C. was supported by the Leukemia and Lymphoma Society Career Development Grant 3160-05.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0831) on May 9, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Carter L. L., Redelmeier T. E., Woollenweber L. A., Schmid S. L. Multiple GTP-binding proteins participate in clathrin-coated vesicle- mediated endocytosis. J. Cell Biol. 1993;120:37–45. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Straubinger R. M., Acton S., Nathke I., Brodsky F. M. 100-kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 1989;86:9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S., McBride H. M., Burgoyne R. D., Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Collins B. M., McCoy A. J., Kent H. M., Evans P. R., Owen D. J. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 2002;156:921–929. doi: 10.1083/jcb.200108123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Regulated portals of entry into the cell. Nature. 2003a;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schroeter T., Schmid S. L. AAK1-mediated μ2 phosphorylation is stimulated by assembled clathrin. Traffic. 2003;4:885–890. doi: 10.1046/j.1398-9219.2003.0142.x. [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Differential requirements for AP-2 in clathrin-mediated endocytosis. J. Cell Biol. 2003b;162:773–780. doi: 10.1083/jcb.200304069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M. J., Yang S., Shang C., Drubin D. G. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J. Cell Biol. 1999;144:1203–1218. doi: 10.1083/jcb.144.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H., Gossen M., Freundlieb S., Bujard H., Schmid S. L. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 1995;257:209–220. doi: 10.1016/s0076-6879(95)57026-8. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Warren R. A., Kessels M. M., Keen J. H., Heuser J., Drubin D. G. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J. Cell Biol. 2001;154:1209–1223. doi: 10.1083/jcb.200106089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C. E., Gibson A., Allchin E. H., Maxwell S., Ruddock L. J., Odorizzi G., Domingo D., Trowbridge I. S., Hopkins C. R. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Kornfeld S. AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J. Cell Biol. 2003;160:699–708. doi: 10.1083/jcb.200211080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener T., Zhao X., Nojima H., Eisenberg E., Greene L. E. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J. Biol. Chem. 2000;275:1365–1370. doi: 10.1074/jbc.275.2.1365. [DOI] [PubMed] [Google Scholar]
- Hannan L. A., Newmyer S. L., Schmid S. L. ATP- and cytosol-dependent release of adaptor proteins from clathrin-coated vesicles: a dual role for Hsc70. Mol. Biol. Cell. 1998;9:2217–2229. doi: 10.1091/mbc.9.8.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. P., Flett A., Smythe C., Hufton L., Wettey F. R., Smythe E. Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor μ2 kinase. J. Cell Biol. 2003;163:231–236. doi: 10.1083/jcb.200304079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. S., Dunn K. W., Pytowski B., McGraw T. E. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor's internalization motif. Mol. Biol. Cell. 1993;4:1251–1266. doi: 10.1091/mbc.4.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyel P. A., Watkins S. C., Traub L. M. Endocytic adaptor molecules reveal an endosomal population of clathrin by total internal reflection fluorescence microscopy. J. Biol. Chem. 2004;279:13190–13204. doi: 10.1074/jbc.M312717200. [DOI] [PubMed] [Google Scholar]
- Kikuno R., Nagase T., Ishikawa K., Hirosawa M., Miyajima N., Tanaka A., Kotani H., Nomura N., Ohara O. Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1999;6:197–205. doi: 10.1093/dnares/6.3.197. [DOI] [PubMed] [Google Scholar]
- Lee D. W., Zhao X., Zhang F., Eisenberg E., Greene L. E. Depletion of GAK/auxilin 2 inhibits receptor-mediated endocytosis and recruitment of both clathrin and clathrin adaptors. J. Cell Sci. 2005;118:4311–4321. doi: 10.1242/jcs.02548. [DOI] [PubMed] [Google Scholar]
- Legendre-Guillemin V., Metzler M., Lemaire J. F., Philie J., Gan L., Hayden M. R., McPherson P. S. Huntingtin interacting protein 1 (HIP1) regulates clathrin assembly through direct binding to the regulatory region of the clathrin light chain. J. Biol. Chem. 2005;280:6101–6108. doi: 10.1074/jbc.M408430200. [DOI] [PubMed] [Google Scholar]
- Manfredi J. J., Bazari W. L. Purification and characterization of two distinct complexes of assembly polypeptides from calf brain coated vesicles that differ in their polypeptide composition and kinase activities. J. Biol. Chem. 1987;262:12182–12188. [PubMed] [Google Scholar]
- Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mayor S., Presley J. F., Maxfield F. R. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. R., Prasad K., Hao W., Augustine G. J., Lafer E. M. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J. Neurosci. 2000;20:8667–8676. doi: 10.1523/JNEUROSCI.20-23-08667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A. M., Berg N., Taylor M. J., Sahlender D. A., Hirst J., Owen D. J., Robinson M. S. Functional analysis of AP-2 α and μ2 subunits. Mol. Biol. Cell. 2006;17:5298–5308. doi: 10.1091/mbc.E06-05-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke I. S., Heuser J., Lupas A., Stock J., Turck C. W., Brodsky F. M. Folding and trimerization of clathrin subunits at the triskelion hub. Cell. 1992;68:899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Ohno H., Stewart J., Fournier M. C., Bosshart H., Rhee I., Miyatake S., Saito T., Gallusser A., Kirchhausen T., Bonifacino J. S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Olusanya O., Andrews P. D., Swedlow J. R., Smythe E. Phosphorylation of threonine 156 of the μ2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Biol. 2001;11:896–900. doi: 10.1016/s0960-9822(01)00240-8. [DOI] [PubMed] [Google Scholar]
- Pagano A., Crottet P., Prescianotto-Baschong C., Spiess M. In vitro formation of recycling vesicles from endosomes requires adaptor protein-1/clathrin and is regulated by rab4 and the connector rabaptin-5. Mol. Biol. Cell. 2004;15:4990–5000. doi: 10.1091/mbc.E04-04-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L., Fava E., Grabner H., Hannus M., Habermann B., Krausz E., Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- Ren M., Xu G., Zeng J., De Lemos-Chiarandini C., Adesnik M., Sabatini D. D. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta D., Conner S. D., Schmid S. L., von Figura K., Honing S. Phosphorylation of the AP2 μ2 subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J. Cell Biol. 2002;156:791–795. doi: 10.1083/jcb.200111068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever S., Damke H., Schmid S. L. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J. Cell Biol. 2000;150:1137–1148. doi: 10.1083/jcb.150.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe D. M., Jesurum A., Murphy R. F. Absence of Na+, K(+)-ATPase regulation of endosomal acidification in K562 erythroleukemia cells. Analysis via inhibition of transferrin recycling by low temperatures. J. Biol. Chem. 1991;266:3469–3474. [PubMed] [Google Scholar]
- Smythe E., Ayscough K. R. The Ark1/Prk1 family of protein kinases. Regulators of endocytosis and the actin skeleton. EMBO Rep. 2003;4:246–251. doi: 10.1038/sj.embor.embor776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W., Oorschot V., Geuze H. J. A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick D. J., Elferink L. A. Rab15 effector protein: a novel protein for receptor recycling from the endocytic recycling compartment. Mol. Biol. Cell. 2005;16:5699–5709. doi: 10.1091/mbc.E05-03-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar F., Bohlander S. K., Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol. Biol. Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E., Harrison S. C., Kirchhausen T. Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc. Natl. Acad. Sci. USA. 2000;97:1096–1100. doi: 10.1073/pnas.97.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda A., Meyerholz A., Ungewickell E. Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. Eur. J. Cell Biol. 2000;79:336–342. doi: 10.1078/S0171-9335(04)70037-0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Ungewickell H., Holstein S. E., Lindner R., Prasad K., Barouch W., Martin B., Greene L. E., Eisenberg E. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- van Dam E. M., Stoorvogel W. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell. 2002;13:169–182. doi: 10.1091/mbc.01-07-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguri S., Dewitte F., Le Borgne R., Rouille Y., Uchiyama Y., Dubremetz J. F., Hoflack B. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol. Biol. Cell. 2003;14:142–155. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B., Steece K. E., Emr S. D. Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J. 1999;18:4383–4393. doi: 10.1093/emboj/18.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettey F. R., Hawkins S. F., Stewart A., Luzio J. P., Howard J. C., Jackson A. P. Controlled elimination of clathrin heavy-chain expression in DT40 lymphocytes. Science. 2002;297:1521–1525. doi: 10.1126/science.1074222. [DOI] [PubMed] [Google Scholar]
- Wilde A., Brodsky F. M. In vivo phosphorylation of adaptors regulates their interaction with clathrin. J. Cell Biol. 1996;135:635–645. doi: 10.1083/jcb.135.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. X., Engqvist-Goldstein A. E., Carreno S., Owen D. J., Smythe E., Drubin D. G. Multiple roles for cyclin g-associated kinase in clathrin-mediated sorting events. Traffic. 2005;6:1103–1113. doi: 10.1111/j.1600-0854.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Gjoerup O., Roberts T. M. The serine/threonine kinase cyclin G-associated kinase regulates epidermal growth factor receptor signaling. Proc. Natl. Acad. Sci. USA. 2004;101:10296–10301. doi: 10.1073/pnas.0403175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.