Abstract
Muscle fiber formation requires the sequential expression of myogenic regulatory factors (MRFs) such as MyoD and myogenin. The messenger RNAs encoding these two proteins are regulated posttranscriptionally through their ability to associate with the RNA-binding protein HuR. HuR localizes first to the nucleus and then to the cytoplasm during muscle differentiation. Therefore, we examined the link between this localization and the promyogenic function of HuR. We show that early in muscle differentiation, HuR is localized to the nucleus of myoblasts by active Transportin 2 (TRN2)-mediated import. In differentiated muscle fibers, however, the TRN2-HuR complex is disrupted, leading to the cytoplasmic localization of HuR, as well as to the stabilization of MyoD and myogenin mRNAs. Interrupting the TRN2-HuR complex using RNA interference against TRN2, or the cell-permeable peptides (AP) fused to the HuR nucleocytoplasmic shuttling domain (HNS), enhanced the efficiency of myofiber formation. Together, our data suggest that HuR import is disrupted in differentiated muscle fibers and this event constitutes an important regulatory step during myogenesis.
INTRODUCTION
In mammals, skeletal muscle tissues are required for vital processes, such as locomotion and breathing (Charge and Rudnicki, 2004). During both embryonic development and the regeneration of muscle upon injury, a process called myogenesis (muscle cell differentiation) is induced to produce muscle fibers (Asakura et al., 2002). Myogenesis involves the sequential up-regulation of the basic helix-loop-helix (bHLH) transcriptional activator proteins of the myogenic regulatory factor (MRF) family: MyoD, Myf5, myogenin, and MRF4 (Charge and Rudnicki, 2004). MyoD and Myf-5 are both expressed in proliferating myoblasts, whereas myogenin and MRF4 are required for maintenance of the differentiation phenotype. Upon activation of the myogenic process, MyoD is up-regulated, and its subsequent collaboration with myogenin leads to the expression of genes necessary for terminal differentiation of muscle tissue fibers (Hasty et al., 1993; Nabeshima et al., 1993; Patapoutian et al., 1995; Rawls et al., 1995; Zhang et al., 1995; Yoon et al., 1997).
One of the interesting features of myogenesis is that once the differentiation process is induced, the expression of MRF proteins is maintained at high levels in myofibers without the need to increase their rates of transcription (Lassar et al., 1991, 1994; Sabourin and Rudnicki, 2000). Therefore, transcription alone is not sufficient to account for the elevated expression of the MRFs in mature myotubes. More recent studies have suggested that posttranscriptional regulatory mechanisms play an important role in MyoD and myogenin synthesis during muscle cell differentiation. In fact, it was shown that the RNA-binding protein HuR is required for the expression, as well as for the stabilization, of MyoD and myogenin transcripts during the differentiation of C2C12 mouse embryonic muscle cells (Figueroa et al., 2003; van der Giessen et al., 2003). HuR binds to its mRNA targets through AU-rich elements (AREs) found in the 3′ untranslated region (3′UTR), affecting the turnover, cellular movements, and translation of the mRNA (Fan and Steitz, 1998b; Gallouzi et al., 2000; Gallouzi and Steitz, 2001; Kullmann et al., 2002; Katsanou et al., 2005). AREs are known to be destabilizing sequences that are present in the 3′UTRs of many short-lived mRNAs, such as cytokines, lymphokines, and proto-oncogenes (Chen and Shyu, 1995). Interestingly, we observed that MyoD and myogenin messages also contain AREs in their 3′UTRs, through which they associate with HuR protein (van der Giessen et al., 2003). Using the RNA interference (RNAi) technique, we demonstrated that depleting the expression of HuR protein in C2C12 cells results in a complete inhibition of myogenesis. The fact that HuR is known to shuttle between the nucleus and the cytoplasm in other cell systems (Brennan et al., 2000; Gallouzi and Steitz, 2001) raises the possibility of a direct link between its cytoplasmic translocation and its promyogenic function.
The cellular movement of HuR is a regulated process involving a nucleocytoplasmic shuttling domain (HuR nucleocytoplasmic shuttling sequence [HNS]; Fan and Steitz, 1998a) and its association with transport receptors such as CRM1 and Transportins 1 and 2 (TRN1 and 2; Brennan et al., 2000; Gallouzi and Steitz, 2001; Guttinger et al., 2004; Rebane et al., 2004). An interesting feature of the HuR protein is its ability to use two alternative pathways to exit from the nucleus to the cytoplasm. One pathway is CRM1-dependent and the other is CRM1-independent involving an unknown export factor (Gallouzi and Steitz, 2001). These observations were obtained upon treatment of cells with antennapaedia (AP) cell-permeable peptides. AP is a short peptide that, when fused to any polypeptides or proteins, facilitates the cellular uptake of the protein chimeras into mammalian cells with high efficiency (>90%). This AP domain was fused to the leucine-rich (NES) CRM1-binding domain or to the HNS motif to study the transport pathways of HuR. These peptides are able to compete for the direct binding of protein receptors, such as TRN1 and TRN2, to HuR through the HNS motif, or for its indirect association with CRM1, involving NES-containing adaptors, such as HuR ligands pp32 and APRIL (Brennan et al., 2000; Gallouzi et al., 2001). It has been shown that, at least in vitro, unlike CRM1 which regulates the nuclear export of HuR, TRN1 and TRN2 HuR import from the cytoplasm to the nucleus (Guttinger et al., 2004; Rebane et al., 2004; Lee et al., 2006). TRN1 and TRN2 are two members of the karyopherin family of transport receptors that specifically bind the HNS region of HuR with different affinities (Guttinger et al., 2004; Rebane et al., 2004). TRN1 and TRN2 seem to be redundant import factors for HuR; however, the functional relevance of HuR-TRN complexes in vivo is still elusive.
The observation that HuR is initially transported to the nucleus in early myogenesis, but later accumulates in the cytoplasm (van der Giessen et al., 2003) where it enhances myogenin and MyoD mRNA stability, suggests that HuR plays a role in the regulation of muscle differentiation. In addition, this indicated that the cellular localization of HuR must also be regulated during myogenesis. To define the link between the nuclear import of HuR and muscle differentiation, we used cell-permeable peptides, RNAi, and immunoprecipitation experiments. The data presented in this study suggest that the disruption of the import of HuR at late stages of myogenesis leads to its cytoplasmic accumulation, as well as to the stabilization of MyoD and myogenin messages, resulting in higher efficiency of muscle differentiation. We also discuss the functional relevance of the HuR-TRN2 association during the myoblast-to-myotube transition.
MATERIALS AND METHODS
Recombinant Protein Production
GST-TRN1 and GST-TRN2 were expressed in BL-21 bacteria by induction with 0.5 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) for 4 h. Recombinant proteins were expressed and prepared as described (van der Giessen et al., 2003).
Cell Culture, Transfection, and Cell-permeable Peptide Treatments
C2C12 cells (ATCC, Manassas, VA, USA) were grown and induced for differentiation as described (van der Giessen et al., 2003). Briefly, differentiation was induced once 100% confluency was reached on cells previously plated on 0.1% gelatin. This was accomplished by replacing growth media with Dulbecco's modified Eagle's medium (DMEM, Sigma, St. Louis, MO) containing 2% horse serum, penicillin/streptomycin antibiotics, 10 μg/ml insulin (Sigma), 10 μg/ml transferring (Sigma), and 50 mM HEPES, pH 7.4 (Invitrogen, Carlsbad, CA). The transfection of small interfering RNA (siRNA) into C2C12 cells was performed as previously described (van der Giessen et al., 2003) with the following modifications. The TRN2 siRNA duplex (Dharmacon, Boulder, CO) aligned with the region corresponding to nucleotides 343–361 of the mouse TRN2 sequence: 5′ ACAGGAGUGUCUCAACAAC 3′. The control siRNA oligonucleotide was previously used as a control duplex (van der Giessen et al., 2003), and the HuR siRNA is the same as previously described (van der Giessen et al., 2003). TRN2 siRNA, control, or mock (transfection reagents only, no oligonucleotide) were transfected as previously described (van der Giessen et al., 2003). Cells that were transfected with both HuR and TRN2 siRNA duplexes were treated with both duplexes together for both transfections. For all transfections, differentiation was induced 24 h after the second RNAi transfection.
For cell-permeable peptide experiments, all peptides (AP-HNS to specifically inhibit HNS-dependent transport, AP-NES to specifically inhibit the CRM1-dependent transport and AP-scHNS, scrambled HNS, as a control peptide) were added to C2C12 myoblasts for two consecutive days: the day before and the day of differentiation induction at a final concentration of 5 μM. For each day of treatment, the peptides were mixed with C2C12 growth or differentiation media (for the first and second day of treatment, respectively) in a microcentrifuge tube, and then 2 ml of the media/peptide solution was added to each well of a six-well plate containing C2C12 myoblasts. The media was changed 24 h after peptide treatment, and cells were allowed to differentiate according to standard protocol.
Production of Polyclonal Anti-TRN2 Antibody
Open Biosystems (Huntsville, AL) searched the mouse TRN2 (Accession Number BC003275) amino acid sequence to predict antigenic peptide sequences; the predicted peptides were compared with other sequences in the NCBI protein database to ensure a specific antibody would be produced. Open Biosystems synthesized a peptide corresponding to amino acids 29–43, with the sequence RIVQDKLKQLNQFPD. The peptide was conjugated to KLH and used to inject rabbits after a 90-d SPF protocol to produce a polyclonal antibody (http://www.openbiosystems.com/antibody.php).
Immunoblotting, Immunofluorescence, and Preparation of Cell Extracts
Total cell extracts were prepared as described (van der Giessen et al., 2003). Western blotting was performed as previously described (Gallouzi et al., 2000). The blots were probed variously with antibodies to HuR (Gallouzi et al., 2000), myoglobin (DAKO, Carpinteria, CA), β-actin (Sigma), TRN1 (Sigma), TRN2, MyoD (BD Biosciences, San Jose, CA), myogenin (BD Biosciences), and hnRNP A1 (Abcam, Cambridge, MA). Immunofluorescence was performed as previously described (van der Giessen et al., 2003).
Subcellular Fractionation
Subcellular fractionation was performed using the PARIS kit from Ambion (Austin, TX) according the manufacturer's instructions. C2C12 cells were treated with TRN2 siRNA as previously described (see above), using 6 × 35-mm wells of each cell treatment for one time point; 36 h after the second transfection, differentiation was induced. Twelve hours after differentiation induction, cells were collected and subjected to subcellular fractionation.
Northern (RNA) Blot Analysis and Actinomycin D Pulse-Chase Experiments
Northern blot analysis was performed using 10–15 μg of total RNA prepared using TRIzol reagent according to the manufacturer's instructions (Invitrogen). After transferring to a Hybond-N membrane (Amersham Biosciences, Piscataway, NJ) and UV cross-linking, the blot was hybridized with MyoD, myogenin, GAPDH, HuR, TRN2, or TRN1 cDNA probes generated by random primer labeling (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. The MyoD, myogenin, and GAPDH probe fragments were made as previously described (van der Giessen et al., 2003). The HuR probe fragment was made by PCR amplification from a GST-HuR plasmid (Di Marco et al., 2005) using the following primers: 5′-gaagaccacatggccgaag-3′ and 5′-cattggtgacggcaccaaac-3′. The TRN2 probe fragment was produced by PCR amplification of TRN2 in a pCMV vector (Open Biosystems, Huntsville, AL) using the following primers: 5′-caacagttctcagagcagt-3′ and 5′-tccgacgatgacgcagac-3′, corresponding to a region of TRN2 located in the 3′UTR that does not recognize TRN1 or any other sequence in the mouse database. The TRN1 probe fragment was made by PCR amplification from a GST-TRN2 plasmid using the following primers: 5′-aggcgattctctgaccagt-3′ and 5′-gtctccaagactgacggac-3′. The MC5 probe was kindly provided by D. Radzioch (McGill University; Radzioch et al., 1987). After hybridization, the membranes were washed and subsequently exposed on BioMax films (Eastman Kodak, Rochester, NY).
The stability of the MyoD and myogenin messages was assessed by treating TRN2 siRNA and control C2C12 cells, at t = 16 h of differentiation, with the RNA pol II inhibitor actinomycin D (ActD; Invitrogen) at a concentration of 5 μg/ml. Total RNA was isolated from the cells at the indicated time points using TRIzol reagent (Invitrogen) and analyzed by Northern blotting.
Immunoprecipitation Analysis
Total cell lysates were prepared from C2C12 cells at indicated time points. Cells were scraped in phosphate-buffered saline (PBS) and centrifuged at 3000 rpm for 5 min at 4°C. The cell pellet was stored at −80°C until all samples had been collected. Total cell extracts were prepared by lysing the cells with RIPA buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.1% SDS) containing complete protease inhibitors (Roche). Lysates were incubated on ice for 15 min, with periodic mixing by vortex, followed by centrifugation at 14,000 rpm for 5 min to pellet the membrane fractions. The supernatant represented the total cell extract. A Bradford protein assay was performed (Bio-Rad, Richmond, CA) to determine the protein concentration of the total lysate for each time point. Equal amounts of total protein (2 mg) for each time point were added to protein A beads (Amersham) previously bound (overnight) to TRN2 or IgG control antibodies (BioCan, Jackson ImmunoResearch Laboratories, West Grove, PA). Cell lysates were incubated with the protein A/antibody mixture for 4 h at 4°C and then washed three times for 3 min with RIPA lysis buffer, followed by several (three times) washes in 1× PBS. The immunoprecipitate was resuspended in 2× Laemmli dye, followed by boiling for 10 min and then vortexing three times for 30 s. The immunoprecipitate was analyzed by Western blotting.
RESULTS
The AP-HNS Cell-permeable Peptide Increases Cytoplasmic HuR Levels and Enhances Muscle Cell Differentiation
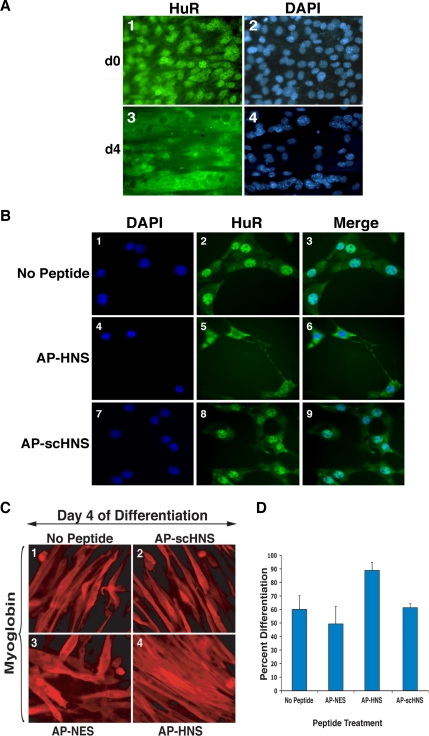
Because HuR is known to shuttle between the nucleus and the cytoplasm in different cell systems (Fan and Steitz, 1998a; Gallouzi and Steitz, 2001), we set out to define the link between its cellular movement and its promyogenic function. Immunofluorescence experiments using anti-HuR antibody and C2C12 cells at the time of differentiation induction (day 0) and late stages of the differentiation process (day 4) confirmed our previous observations (van der Giessen et al., 2003) that HuR protein accumulates in the cytoplasm of mature myotubes (Figure 1A). Because we did not observe any significant nuclear reaccumulation of HuR at late stages of myogenesis, our results suggested that the nuclear import of HuR had been interrupted in myofibers. Moreover, these data raised the possibility that interfering with the nuclear import of HuR at earlier stages of myogenesis could activate myotube formation. To test this possibility, we used the cell-permeable peptide inhibitors AP-HNS and AP-NES (Gallouzi and Steitz, 2001). In HeLa cells AP-NES specifically inhibited CRM1-dependent HuR export, whereas AP-HNS affected its HNS-dependent transport (Gallouzi and Steitz, 2001; Flint et al., 2005). Because these permeable peptides have a long half-life (>12 h; Derossi et al., 1998) and are also known to facilitate the cellular uptake of any polypeptide with a high efficiency (>90%; van der Giessen et al., 2003; Xu et al., 2005), they were the perfect tool to interfere with HuR cellular movement during muscle differentiation. We first tested the effect of these peptides on the cellular distribution of HuR in undifferentiated C2C12 cells. Exponentially growing myoblasts were treated with these peptides for 16 h, and then the cellular localization of HuR was determined with the anti-HuR monoclonal antibodies (Gallouzi et al., 2000). AP-HNS, but not AP-scHNS (scrambled HNS), caused cytoplasmic sequestration of HuR in C2C12 cells (Figure 1B, compare panels 5 and 6 to panels 2, 3, 8, and 9). AP-NES, however, did not have any significant effect on HuR cellular localization (Supplementary Figure 1). Because the HNS motif is reported to regulate the nucleocytoplasmic shuttling of HuR in other cell systems (Fan and Steitz, 1998a) and AP-HNS blocked HuR import in myoblasts in the current study (Figure 1B), we concluded that the main function of this domain in muscle cells is to mediate the association between HuR and its import receptor(s).
Figure 1.
The AP-HNS cell-permeable peptide enhances muscle cell differentiation and increases the accumulation of HuR in the cytoplasm. (A) C2C12 cells grown to confluency (d0) or induced to differentiate for 4 d (d4) were fixed and used for immunofluorescence, using the monoclonal anti-HuR antibody. DAPI staining was used to visualize the nuclear compartment. DAPI and HuR images of a single representative field of view are shown for each time point. (B) The subcellular localization of HuR after treatment of exponentially growing C2C12 cells with AP-HNS, AP-scHNS (scrambled HNS) or no peptide was analyzed. 24 h after peptide treatment. Myoblast cells were fixed, and the localization of endogenous HuR was determined by double immunofluorescence staining using a monoclonal anti-HuR antibody and DAPI. DAPI, HuR, and a merged image of a single representative field of view are shown for each treatment. (C) Exponentially growing C2C12 cells were treated with AP-conjugated peptides for 24 h, and differentiation was induced by serum starvation. AP-conjugated peptides were added with differentiation medium for another 24 h. Four days after differentiation induction (day 4), cells were fixed and stained for myoglobin. The degree of differentiation after each peptide treatment was analyzed by immunofluorescence. Myoglobin-stained images of a single representative field of view for each peptide treatment are shown. (D) Immunofluorescence staining with the anti-myoglobin antibody was used to calculate the fusion index of the myotubes after each peptide treatment. The quantitative analysis of differentiation was performed by determining the number of nuclei in each microscopic field in relation to the number of nuclei in myotubes in the same field. The percentage of differentiation is indicated. Error bars, SD of two independent experiments.
Our data suggested that the cytoplasmic accumulation of HuR could represent an important regulatory step during the myogenic process. If this hypothesis is true, treating exponentially growing myoblasts with AP-HNS but not with the AP-NES peptide should induce an early cytoplasmic accumulation of HuR and a significant increase in the degree of myotube formation. To test this possibility, we treated C2C12 cells with AP-HNS, AP-NES, or AP-scHNS peptides the day before and the day of differentiation induction and then fixed cells for immunofluorescence on day 4 of myogenesis. As predicted, the staining of the myogenic marker myoglobin (van der Giessen et al., 2003) increased by 30% in the C2C12 cells treated with AP-HNS compared with those treated with the AP-NES or the controls (AP-scHNS or no peptides; Figure 1C, compare panel 4 to panels 1, 2, and 3 and Figure 1D). Although at day 2 of differentiation a slight enhancement of the differentiation phenotype has been observed in AP-HNS–treated C2C12 cells compared with the controls (Supplementary Figure 2), the effect of this cell-permeable peptide reaches its maximum between day 3 and 4 of the differentiation process (Figure 1D). Furthermore, treating C2C12 cells with both AP-NES and AP-HNS peptides did not have any visible effect on muscle differentiation efficiency (Figure 1D). These results indicated that interfering with the nuclear import of HuR, but not with its CRM1-mediated cellular movement, correlated with enhanced muscle cell differentiation.
HuR Nuclear Import at Early Stages of Muscle Cell Differentiation Is Mediated by the TRN2 Import Receptor
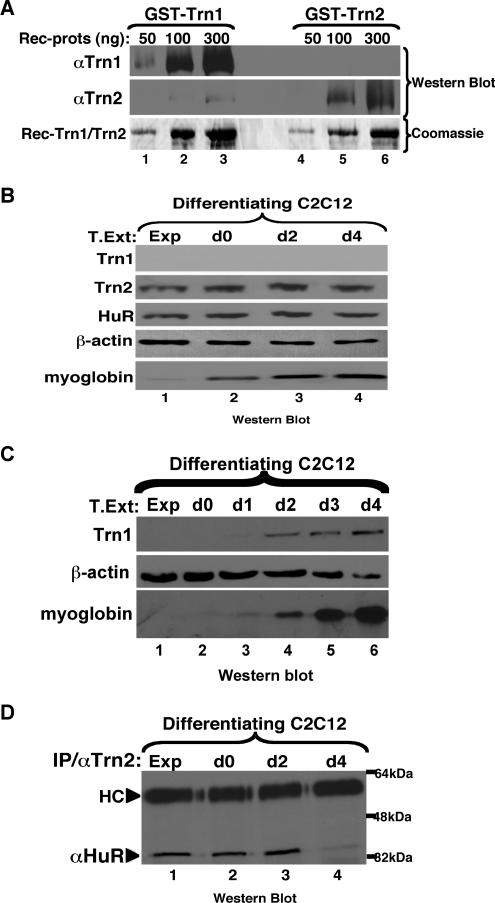
HuR movement from the cytoplasm to the nucleus is mediated in other cell systems by two alternate import factors, TRN1 and TRN2 (Gallouzi and Steitz, 2001; Guttinger et al., 2004; Rebane et al., 2004). It is therefore possible that, in the experiment described above, the AP-HNS peptide interrupted HuR-TRN1 and/or -TRN2 complexes, leading to the observed increase in the efficiency of myotube formation. As a first step in defining the regulation of HuR nuclear import in myogenesis, we examined the expression of TRN1 and TRN2 in C2C12 cells undergoing differentiation. Because only anti-TRN1 antibodies were commercially available, we generated an anti-TRN2 polyclonal antibody and confirmed that the anti-TRN2 antibody recognized TRN2 protein with high specificity (Figure 2A). To define the expression profile of TRN1 and TRN2 during myogenesis, total cell extracts from differentiating C2C12 cells were prepared and analyzed by Western blot using the anti-TRN1, -TRN2, and -HuR antibodies. TRN2 and HuR were highly expressed during all myogenic stages when 30 μg of total cell lysate was migrated on the SDS-polyacrylamide gel (Figure 2B). On the other hand, detection of TRN1 required 100 μg of cell lysate, with a weak band corresponding to TRN1 that was observed only in mature myotubes (Figure 2C). These results suggested that TRN2 is likely to be the main import factor of the HuR protein at early stages of myogenesis.
Figure 2.
HuR and TRN2 are expressed during myogenesis, and associate until day 2 of differentiation. (A) Fifty, 100, and 300 ng of recombinant GST-TRN1 and GST-TRN2 were run on SDS-PAGE and subjected to Western blot analyses using antibodies against TRN1 and TRN2. SDS-PAGE followed by Coomassie staining of 1, 3, and 10 μg of recombinant protein demonstrates there was protein loaded in all lanes. (B) Western blot analyses were performed using 30 μg of total protein extracts from exponentially growing (Exp) and differentiating (day 0, d0, to day 4, d4) C2C12 cells. The blots were probed with antibodies to TRN1, TRN2, HuR, myoglobin, and β-actin. (C) Western blot analyses were performed using 100 μg of total protein extracts from exponentially growing (Exp) and differentiating (day 0, d0, to day 4, d4) C2C12 cells in order to detect the TRN1 protein. The blot was probed with antibodies to TRN1, myoglobin, and β-actin. (D) Total cell extracts were prepared from C2C12 cells on the indicated days during myogenesis and subjected to immunoprecipitation using a polyclonal α-TRN2 antibody. The immunoprecipitates were run on an SDS-PAGE, and a monoclonal anti-HuR antibody was used in Western blotting to look for association between the two proteins. HC refers to the heavy chain of the TRN2 antibody used for the IP.
The fact that HuR accumulates in the cytoplasm between day 3 and 4 during myogenesis (Figure 1A), and van der Giessen et al. (2003) suggested that the import of HuR was interrupted in mature myotubes. Therefore, we followed the association between HuR and TRN2 during the myogenic process. Total extracts from C2C12 cells undergoing differentiation were prepared and used for coimmunoprecipitation experiments with anti-TRN1 and -TRN2 antibodies. Western blot analysis showed that at early stages of myogenesis, HuR coimmunoprecipitated with TRN2 (Figure 2D) but not with TRN1 (data not shown). However, at day 4 of the differentiation process association between TRN2 and HuR was reduced by 10-fold compared with early stages of myogenesis (Figure 2D, compare lane 4 to lanes 1–3). The levels of HuR and TRN2 proteins remained unchanged during myogenesis (Figure 2B), suggesting that the significant reduction in the association between these proteins is not due to a reduction in their synthesis. Together our observations indicated that the accumulation of HuR in the cytoplasm of mature myotubes correlated with a reduction in its ability to associate with the import factor TRN2. This data also suggested that although the differential expression of TRN1 observed in myotubes could indicate its implication in myogenesis (Figure 2C), it is likely that this function does not involve the HuR protein.
RNAi-mediated TRN2 Depletion Leads to the Enhancement of the Efficiency of Muscle Cell Differentiation
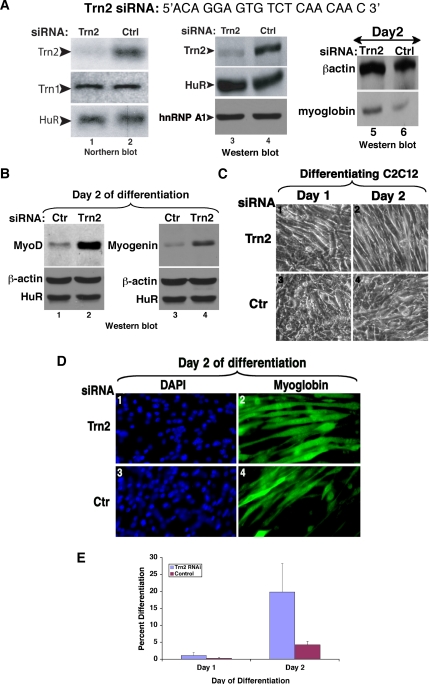
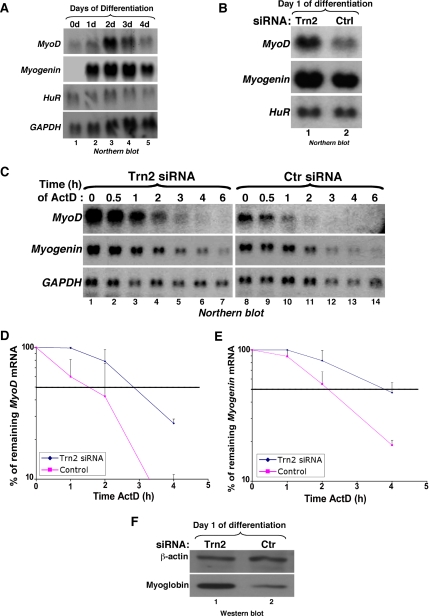
To determine if TRN2 plays a critical role in C2C12 differentiation, an siRNA duplex (TRN2-siRNA) was designed to target the 5′ region of the TRN2 message in order to knockdown the TRN2 mRNA (Figure 3A). The negative control (Ctrl) used was a sequence previously shown to have no effect on C2C12 mRNAs (van der Giessen et al., 2003). Two successive transfections (each one separated by 24 h) of siRNA duplexes were necessary to achieve an efficient and specific knockdown of TRN2 (see Materials and Methods). Using TRN2-siRNA, more than 75% reduction in TRN2 levels was achieved as shown by both Northern and Western blotting (Figure 3A, lanes 1–4). Depletion of TRN2 mRNA led to a threefold increase in the level of myoglobin protein (Figure 3A, lanes 5 and 6), suggesting an enhancement in muscle cell differentiation. This result was confirmed when we verified the effect of the TRN2-siRNA duplexes on the protein expression levels of the myogenic transcription factors MyoD and Myogenin. We showed that muscle cells depleted of TRN2 protein express four and threefold more MyoD and myogenin protein, respectively, than the controls at day 2 after differentiation initiation (Figure 3B). Likewise, in agreement with the protein expression results, we observed a fourfold increase in the number of myotubes in C2C12 cells treated with TRN2-siRNAs compared with the controls (Figure 3, C–E). These data showed that depleting the expression of TRN2 alone is sufficient to enhance muscle cell differentiation.
Figure 3.
Knockdown of Transportin 2 leads to enhanced myogenesis (A) The TRN2 siRNA duplex (Dharmacon) targets the TRN2 mRNA near the 5′ end of the message. A nonspecific control oligonucleotide was used as a negative control. Northern blot analysis was performed on 10 μg of total RNA isolated on day 0 of differentiation [48 h after transfection of siRNA duplexes (lanes 1–2)]. The membrane was probed with specific 32P-radiolabeled probes against TRN2, TRN1, and HuR (lanes 1 and 2). Western blot analysis was performed on 15 μg of total cell extract at the same time point (lanes 3 and 4) or at day 2 of differentiation (lanes 5 and 6). The membrane was probed with antibodies to TRN2, HuR, hnRNP A1 (lanes 3 and 4) or to myoglobin and β-actin (lanes 5 and 6) to determine the degree of differentiation after the siRNA treatments. (B) Total cell extracts from TRN2 siRNA- and control (ctr)-treated C2C12 cells were prepared on day 2 of differentiation. Western blotting using antibodies to MyoD (lanes 1 and 2) and myogenin (lanes 3 and 4) demonstrated increased expression of these MRFs in the absence of TRN2 compared with the control. (C) C2C12 myoblasts were transfected with TRN2 or Ctrl siRNA oligonucleotides, and differentiation was induced when cells reached 100% confluency. Phase-contrast pictures of a single representative field of view for each cell treatment on day 1 and day 2 of differentiation are shown. (D and E) TRN2 siRNA-treated and control cells were fixed, and immunofluorescence staining against myoglobin and DAPI was performed on C2C12 cells on day 2 of differentiation. (D) A single representative field of view for each cell treatment is shown. (E) The fusion index is shown and was calculated as described (Figure 1). Error bars, SD of three independent experiments.
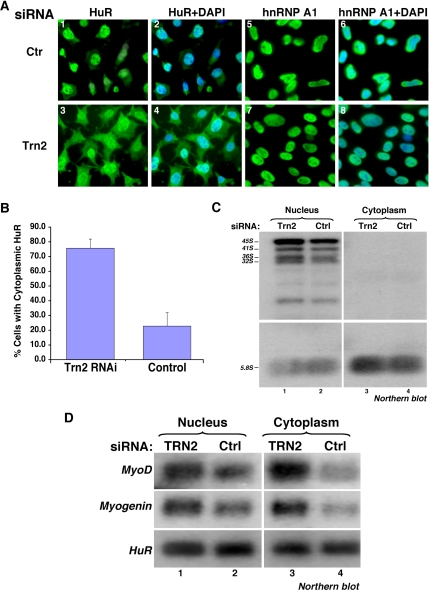
Silencing TRN2 Expression Results in Cytoplasmic Accumulation of HuR and Stabilization of MyoD and Myogenin mRNAs
HuR was previously shown to be required for myogenesis due, in part, to its ability to associate with and stabilize key MRF messages such as MyoD and myogenin (Figueroa et al., 2003; van der Giessen et al., 2003). The cellular localization where this occurs, however, is unknown. To address this question, we first tested the effect of TRN2 on the cellular localization of HuR and its mRNA targets during muscle differentiation. C2C12 cells were treated with TRN2-siRNA, and the cellular distribution of HuR protein was assessed using immunofluorescence. We observed that the cytoplasmic localization of HuR, but not of hnRNP A1 protein (another known TRN2 ligand; Guttinger et al., 2004; Rebane et al., 2004), increased by threefold in myoblasts treated with TRN2-siRNA compared with the control (Figure 4A, compare panels 1 and 3 with panels 5 and 7, and 4B). This observation suggested that although TRN2 has been shown to affect the cellular movement of other regulatory proteins (Lee et al., 2006), one of its main functions in muscle cells is to mediate the import of HuR from the cytoplasm to the nucleus. To assess the effect of TRN2 depletion on the cellular distribution of MyoD and myogenin mRNAs, C2C12 cells treated with TRN2- or Ctr-siRNAs were induced for differentiation. Twelve hours after induction, the cells were harvested and mRNA was extracted from nuclear and cytoplasmic fractions. Northern blot analysis using specific radiolabeled probes showed a fourfold increase in the cytoplasmic levels of MyoD and myogenin mRNAs in TRN2-depleted cells compared with the control, whereas their nuclear levels increased by only twofold (Figure 4D). Because we did not observe any cross-contamination between the two fractions at the RNA (Figure 4C) or the protein (data not shown) levels, we concluded that both MyoD and myogenin messages follow the cellular movement of HuR protein during muscle differentiation. These results suggested that disrupting the TRN2-HuR complex could represent a regulatory step that promotes the cytoplasmic localization and the expression of MyoD and myogenin mRNAs.
Figure 4.
SiRNA-mediated Trn2 knockdown leads to the cytoplasmic accumulation of HuR protein and MyoD and myogenin mRNAs. (A) Immunofluorescence staining, using DAPI and a monoclonal HuR antibody, or a monoclonal hnRNP A1 antibody and DAPI, was performed on cells treated with TRN2 or control (Ctr) RNAi duplexes to look at the localization of HuR and hnRNP A1 in the presence and absence of TRN2. A single representative field of view for each cell treatment (HuR and DAPI or hnRNP A1 and DAPI) is shown. (B) The percent of transfected cells with cytoplasmic accumulation of HuR was determined by counting the total number of cells in each field of view and dividing by the number of cells with predominantly cytoplasmic HuR. Error bars, SD of two independent experiments. (C) Northern blotting was used to confirm the integrity of cellular fractionation of RNA components at 12 h of differentiation. A 32P-radiolabeled MC5 probe recognizes pre-rRNA, present only in the nucleus of cells, and is used as a marker to confirm that there is no nuclear contamination in the cytoplasmic fractions. The 5.8S (processed) RNA is observed in both nuclear and cytoplasmic compartments. (D) RNA isolated from nuclear and cytoplasmic cellular compartments was used for Northern blotting. Radiolabeled probes for MyoD and myogenin messages were used to determine the relative abundance of each message in each cellular compartment in the TRN2 siRNA compared with control-treated cells. HuR serves as a loading control, as its mRNA cellular localization and expression levels do not change in the presence or absence of TRN2.
It has been shown that during the terminal phase of muscle differentiation, the up-regulation of MyoD and myogenin proteins and mRNAs correlates with HuR accumulation in the cytoplasm (Figueroa et al., 2003; van der Giessen et al., 2003). Therefore, to establish the link between the interruption of HuR nuclear import and the promyogenic function of HuR, we followed the expression of MyoD and myogenin messages in the absence or presence of TRN2 protein. It is well established that the induction of MyoD and myogenin expression occurs at different times during myogenesis (Charge and Rudnicki, 2004). To define the time at which both MyoD and myogenin mRNAs are expressed during myogenesis, we performed a Northern blot analysis using total mRNAs prepared from differentiating C2C12 cells and radiolabeled probes against MyoD, myogenin, GAPDH, and HuR messages. We observed that although only MyoD is expressed at day 0, both MyoD and myogenin messages are detected at day 1 after differentiation initiation (Figure 5A). Furthermore, the steady state levels of these two mRNAs increased by twofold in differentiated C2C12 cells lacking TRN2 protein compared with controls (Figure 5B).
Figure 5.
TRN2 knockdown increases the steady state levels and stability of MyoD and myogenin mRNAs. (A) Northern blotting was performed on total mRNA that was isolated from C2C12 cells on each day of differentiation using radiolabeled probes against MyoD, Myogenin, HuR, and GAPDH messages. (B) Total RNA was isolated from TRN2 or control (Ctrl) siRNA-treated C2C12 cells, and Northern blotting was performed using specific 32P-labeled cDNA probes for MyoD, myogenin, and HuR messages to assess steady state levels of MyoD and myogenin mRNAs. (C) The stability of MyoD and myogenin mRNA was assessed in TRN2 siRNA- and control-treated cells by adding the transcriptional inhibitor actinomycin D (ActD) 16 h after differentiation induction and harvesting RNA at the indicated time points after ActD treatment for Northern blotting. GAPDH was used as a loading control. (D and E) The stability of the MyoD (D) and myogenin (E) mRNAs in cells treated with TRN2 and control siRNAs was determined and quantified from Northern blotting using the Image Quant computer program (Molecular Dynamics, Sunnyvale, CA) to measure band intensities. The percent remaining of both mRNAs was defined as the relative intensity of each message to GAPDH bands at each time point and was calculated as a percent of the abundance of each message at 0 h of ActD treatment and plotted on a logarithmic curve. The dotted horizontal line represents 50% remaining mRNA relative the original abundance of the message and therefore the half-life of the message under the different cell treatments. Error bars, SD of two independent experiments. (F) Total cell extracts were prepared from C2C12 cells at t = 16 h of differentiation (day 1), the same time point used for ActD treatment, and SDS-PAGE was performed for Western blot analysis using antibodies for myoglobin and β-actin.
This increase in mRNA abundance was consistent with the observed increase in the protein levels of these two MRFs, as well as with an increase in the differentiation efficiency as described above (Figure 3, B–E). To investigate the functional link between the disruption of the TRN2-HuR complex and a potential increase in the half-life of MyoD and myogenin mRNAs, we performed ActD (specific inhibitor of polII transcription; Di Marco et al., 2005) pulse-chase experiments on differentiating C2C12 cells depleted of TRN2 protein. Total RNAs were collected during the 6 h of ActD treatment and used for Northern blot analysis. The half-lives of MyoD and myogenin mRNAs increased by about twofold (Figure 5, C–E). Furthermore, the same stabilization effect of these messages was also observed in C2C12 cells treated with AP-HNS, but not with control cell-permeable peptides (data not shown). The enhanced differentiation of the TRN2-depleted C2C12 cells used for these experiments was confirmed, this time by Western blotting using an antibody against the differentiation marker myoglobin (Figure 5F). In the ActD pulse-chase experiment, the level of total mRNA of both MyoD and myogenin messages at time 0 in RNAi-treated cells was twofold higher than in the control (Figure 5C, compare lanes 1 and 8), which was consistent with the observed increase in their steady state levels (Figure 5B). However, the rate of decay measured was independent of the amount of mRNA used and rather reflected the rate at which a message was degraded. In fact, the half-life of these mRNAs was calculated as a percentage of the remaining transcripts at any given time point relative to the 0h time point, normalized to the amount of GAPDH mRNA. This stabilization effect corresponded to an increase in the protein expression levels of both the MyoD and myogenin (Figure 3B). Therefore, our results indicated that the stabilization of MyoD and myogenin mRNAs in muscle cells could be attributed to the cytoplasmic accumulation of HuR, which was mediated by the disruption of TRN2-HuR complex.
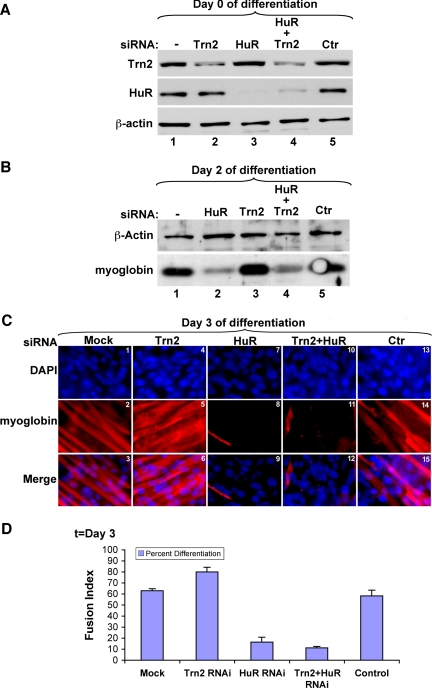
We have previously shown that the knockdown of HuR in undifferentiated C2C12 cells prevented them from entering the myogenic process (van der Giessen et al., 2003). Therefore, if the enhanced efficiency of myogenesis observed under TRN2-depleted conditions is due to its subsequent effect on the subcellular localization of HuR, the double knockdown of both TRN2 and HuR should result in a defect in the myogenic process. To test this possibility, we knocked down both HuR and TRN2 proteins from myoblasts using siRNAs (HuR-siRNA; van der Giessen et al., 2003; and TRN2-siRNA; Figure 3) and then monitored differentiation efficiency. The depletion of both HuR and TRN2 proteins was confirmed by Western blot analysis using the anti-TRN2 and -HuR antibodies (Figure 6A). The same approach was used to confirm the effect of the depletion of each protein individually on myogenesis. We followed the expression of the differentiation marker myoglobin at day 2 after differentiation induction in C2C12 cells treated with TRN2- and HuR-siRNAs either individually or together. As expected, the expression levels of myoglobin protein significantly decreased in cells treated with HuR-siRNA, whereas a twofold increase was observed in cells treated with TRN2-siRNA (Figure 6B, compare lanes 2 and 3 to lane 1). However, the expression levels of myoglobin protein remained significantly low in cells treated with both TRN2- and HuR-siRNAs (Figure 6B, lane 4). This observation was confirmed using immunofluorescence staining for DAPI and myoglobin protein for the same cell treatments. We observed a significant decrease in the number of visible myotubes in HuR/TRN2-depleted cells compared with the controls (Figure 6C, compare panels 11 and 12 with panels 2, 3, 14, and 15, and 6D). Together our results suggested that the positive effect of the depletion of TRN2 protein on the myogenic process involves the disruption of its association with the HuR protein.
Figure 6.
Double knockdown of HuR and Transportin 2 prevented myogenesis. (A) C2C12 cells were transfected with the HuR siRNA duplex, targeting the HuR mRNA at the 111–131 sequence, the Trn2 siRNA duplex (Figure 3), or a combination of both, as well as a control siRNA duplex (Ctr) or transfection reagents only (−). Total cell extracts were prepared 24 h after transfection, and Western blotting was performed using antibodies to Trn2, HuR, and β-actin to demonstrate the degree of knockdown of each (HuR and Trn2) protein. (B) Western blot analysis of 20 μg of total cell extracts harvested on day 2 of differentiation was performed, and the membrane was probed with antibodies to myoglobin and β-actin. (C) C2C12 cells that were transfected with siRNA (transfection reagents only [mock] Trn2, HuR, Trn2+HuR, and control) were fixed on day 3 of differentiation. Immunofluorescence staining with anti-myoglobin was performed to determine the differentiation status of the transfected cells. DAPI, myoglobin, and merged (DAPI+myoglobin) images from a single representative field of view are shown. (D) The fusion index of myotubes was calculated to assess the percentage of differentiation in C2C12 cells under each transfection condition. The quantitative analysis was performed as described in Figure 1. Error bars, SD of two independent experiments.
DISCUSSION
The data presented in this study demonstrate that TRN2-mediated HuR import plays a crucial role in maintaining HuR levels in the nucleus during the early steps of muscle differentiation. However, once myotubes are formed, this import activity seems to be interrupted (Figure 2D), leading to cytoplasmic accumulation of HuR protein (Figures 1A and 4A) as well as the stabilization of the MyoD and myogenin messages (Figure 5). These results are consistent with our previous observations showing that HuR does not directly associate with the MyoD mRNA at early stages of myogenesis; however, in mature myofibers, HuR associates with and stabilizes both MyoD and myogenin messages (Figueroa et al., 2003; van der Giessen et al., 2003). Together, our observations suggest a model whereby TRN2-mediated HuR import is disrupted in mature myotubes, and this event constitutes an important regulatory step during myogenesis.
It has previously been shown that the expression of the MRFs (proteins and mRNAs) must be maintained at high levels in myofibers without a constant increase in their rates of transcription (Figueroa et al., 2003; Charge and Rudnicki, 2004), implicating the role of posttranscriptional regulation. At the induction step of the myogenic process, there is a requirement for the rapid and sequential up-regulation of both MyoD and myogenin mRNAs (Charge and Rudnicki, 2004). Our cellular fractionation experiments followed by Northern blotting (Figure 4), combined with previous actinomycin D pulse-chase experiments (Figueroa et al., 2003), suggest that HuR could be required for the rapid export and subsequent stabilization of both MyoD and myogenin messages at the onset of differentiation. Later in the differentiation process, however, once myotubes are formed, there is accumulation of MyoD and myogenin messages in the cytoplasm, ensuring the maintenance of the differentiated myotubes. Thus, even though their rapid nuclear export may no longer be required, they must still be protected from the cytoplasmic mRNA decay machinery. Indeed, it has been suggested that the KH-domain binding protein KSRP recruits the decay machinery leading to the destabilization of MyoD and myogenin messages in undifferentiated cells (Briata et al., 2005). Our data, however, show that a high expression level of these two messages is maintained throughout myogenesis (Figure 5A). This argues that the requirement for the concurrent stabilization of these messages is accomplished, as our data suggest (Figure 5, C–E), by HuR. Therefore, it is likely that HuR plays a critical role in both the initiation and the maintenance of C2C12 differentiation.
Previous work from several groups has demonstrated that TRN2 association with HuR is mediated by the HNS motif (Gallouzi and Steitz, 2001; Guttinger et al., 2004; Rebane et al., 2004). When we introduced this motif in muscle cells using the cell-permeable peptide AP-HNS, we interrupted the import of HuR (Figure 1B) and enhanced the efficiency of the myogenic process (Figure 1, C and D), without affecting the cellular levels of TRN2 and HuR proteins (data not shown). These results clearly indicate that the role of TRN2 in muscle differentiation is intimately linked to its ability to interact with HNS-containing proteins such as HuR. Because it is possible that C2C12 cells also express other proteins containing HNS-like motifs, we can assume that these factors, if they exist, could affect TRN2 function in myogenesis. To explore this possibility, we performed a BLAST search and alignment of both mouse and human databases using the HNS sequence. We observed that HuR and the other three ELAV proteins, HuB, HuC, and HuD, are the only known HNS-containing proteins (data not shown). However, because the three other ELAV proteins (HuB, HuC, and HuD) are expressed only in the brain (Keene, 1999), we believe that in muscle cells the AP-HNS peptide specifically inhibited the association between HuR and TRN2.
The increased stability of the MyoD and myogenin messages could account for the enhanced differentiation observed as a result of C2C12 treatment with AP-HNS (Figure 1) or TRN2 knockdown (Figure 3). These treatments also enhance the level of HuR in the cytoplasm, therefore providing a strong argument for a direct link between two phenotypes: mRNA stability and HuR/mRNP (HuR with its mRNA targets) cellular localization. These observations, combined with the inhibited myogenic phenotype observed in the HuR/TRN2 double knockdown experiment (Figure 6), support a model in which TRN2 is required for the regulation of HuR subcellular localization, which in turn is required for the regulation of MRF mRNA levels.
Our data showing that HuR is maintained in the nucleus of myoblasts and then translocated to the cytoplasm of myofibers could suggest that its promyogenic function is required in both compartments at different stages of myogenesis. The possibility of a nuclear function of HuR at early stages of muscle differentiation was previously suggested from experiments showing that depleting HuR protein from undifferentiated myoblasts, when it is mainly localized to the nucleus, led to a significant decrease in the expression of MyoD mRNA (van der Giessen et al., 2003). These observations could indicate that HuR protects MyoD mRNA from decay in the nucleus at early stages of muscle differentiation. However, the fact that HuR is known to shuttle between the nucleus and the cytoplasm in other cell systems (Fan and Steitz, 1998a) also suggests that, in undifferentiated myoblasts and early in the differentiation process, HuR could act as a stabilizer for MyoD mRNA by translocating to the cytoplasm, even if briefly. This possibility is supported by experiments described in this article showing that AP-HNS treatment (Figure 1) and TRN2 depletion (Figure 3) lead to an earlier cytoplasmic localization of HuR, as well as to an increase of the cytoplasmic levels of MyoD and myogenin messages (Figure 4). This, in turn, activates myogenesis and stabilizes both of these MRF messages (Figures 3 and 5). This cytoplasmic role of HuR is consistent with the fact that myogenin mRNA and protein are expressed only once myoblasts stop dividing and fuse to form myotubes (Figure 5A; Charge and Rudnicki, 2004). Therefore, the cytoplasmic translocation of HuR during muscle fiber formation is likely to be one of the mechanisms by which the cell protects myogenin mRNA from decay, leading to its rapid accumulation.
Overall, our data indicate that TRN2-mediated nuclear import of HuR is an important step that regulates the abundance of HuR in the cytoplasm of differentiating myotubes. These data also suggest that the levels of HuR in the cytoplasm must be under tight regulation to maintain the expression of MRF mRNAs (MyoD and moyogenin) at the levels that is required for normal differentiation. It is possible that posttranslational modifications of HuR or TRN2, perhaps induced by extracellular signals, alter the association of these proteins. It has been shown that the HuR protein is methylated on a specific arginine residue (R217) by the protein arginine methyltransferase CARM1 (Li et al., 2002). Additionally, a recent report has suggested that under oxidative stress, HuR can be phosphorylated on its amino acid residues ser88, ser100, and Tyr118 by the cell cycle checkpoint kinase Chk2 (Abdelmohsen et al., 2007). It will be of high interest to assess the effect of these posttranslational modifications on the cellular movement of HuR during myogenesis. Addressing this question will provide additional insight into how muscle cells integrate extracellular stimuli to engage the differentiation process. It is possible that other posttranslational modifications on HuR or TRN2 occur in muscle cells, which affect when the two proteins should bind and when they should dissociate. The answers to these mechanistic questions are currently unknown. Therefore, defining the regulatory mechanisms that mediate the transport of HuR via a particular pathway in muscle cells will help better understand its promyogenic function.
Supplementary Material
ACKNOWLEDGMENTS
We particularly thank Dr. S. Di-Marco for his help with the Northern blots for MyoD and myogenin mRNAs as well as for advices and discussions. We are grateful to Drs. V. Dormoy-Raclet and R. Mazroui, and C. von Roretz and J. Behrmann for helpful discussions and comments on the manuscript. We are grateful to Dr. N. Sonenberg for numerous suggestions and comments on different aspects of this work. This work was supported by a McGill Studentship award to K.V. and a Canadian Institutes for Health Research operating grant Mop-57680 and an Fonds de la Recherche en Santé du Québec “Subvention d'etablissement de jeune chercheur” Frsq 23516-2760 to I.G. I.G. is a recipient of TierII Canada Research Chair.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0167) on May 2, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Abdelmohsen K., et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol. Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura A., Seale P., Girgis-Gabardo A., Rudnicki M. A. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. M., Gallouzi I. E., Steitz J. A. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J. Cell Biol. 2000;151:1–14. doi: 10.1083/jcb.151.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briata P., Forcales S. V., Ponassi M., Corte G., Chen C. Y., Karin M., Puri P. L., Gherzi R. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell. 2005;20:891–903. doi: 10.1016/j.molcel.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Charge S. B., Rudnicki M. A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Shyu A. B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Derossi D., Chassaing G., Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- Di Marco S., Mazroui R., Dallaire P., Chittur S., Tenenbaum S. A., Radzioch D., Marette A., Gallouzi I. E. NF-(kappa)B-mediated MyoD decay during muscle wasting requires nitric oxide synthase mRNA stabilization, HuR protein, and nitric oxide release. Mol. Cell. Biol. 2005;25:6533–6545. doi: 10.1128/MCB.25.15.6533-6545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. C., Steitz J. A. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl. Acad. Sci. USA. 1998a;95:15293–15298. doi: 10.1073/pnas.95.26.15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. C., Steitz J. A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998b;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa A., Cuadrado A., Fan J., Atasoy U., Muscat G. E., Munoz-Canoves P., Gorospe M., Munoz A. Role of HuR in skeletal myogenesis through coordinate regulation of muscle differentiation genes. Mol. Cell. Biol. 2003;23:4991–5004. doi: 10.1128/MCB.23.14.4991-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Huang W., Goodhouse J., Kyin S. A peptide inhibitor of exportin1 blocks shuttling of the adenoviral E1B 55 kDa protein but not export of viral late mRNAs. Virology. 2005;337:7–17. doi: 10.1016/j.virol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Gallouzi I. E., Brennan C. M., Steitz J. A. Protein ligands mediate the CRM1-dependent export of HuR in response to heat shock. RNA. 2001;7:1348–1361. doi: 10.1017/s1355838201016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallouzi I. E., Brennan C. M., Stenberg M. G., Swanson M. S., Eversole A., Maizels N., Steitz J. A. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc. Natl. Acad. Sci. USA. 2000;97:3073–3078. doi: 10.1073/pnas.97.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallouzi I. E., Steitz J. A. Delineation of mRNA export pathways by the use of cell-permeable peptides. Science. 2001;294:1895–1901. doi: 10.1126/science.1064693. [DOI] [PubMed] [Google Scholar]
- Guttinger S., Muhlhausser P., Koller-Eichhorn R., Brennecke J., Kutay U. Transportin2 functions as importin and mediates nuclear import of HuR. Proc. Natl. Acad. Sci. USA. 2004;101:2918–2923. doi: 10.1073/pnas.0400342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J. H., Edmondson D. G., Venuti J. M., Olson E. N., Klein W. H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Katsanou V., Papadaki O., Milatos S., Blackshear P. J., Anderson P., Kollias G., Kontoyiannis D. L. HuR as a negative posttranscriptional modulator in inflammation. Mol. Cell. 2005;19:777–789. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Keene J. D. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA. 1999;96:5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann M., Gopfert U., Siewe B., Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev. 2002;16:3087–3099. doi: 10.1101/gad.248902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991;66:305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Skapek S. X., Novitch B. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr. Opin. Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Lee R. C., Hammell C. M., Ambros V. Interacting endogenous and exogenous RNAi pathways in Caenorhabditis elegans. RNA. 2006;12:589–597. doi: 10.1261/rna.2231506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Park S., Kilburn B., Jelinek M. A., Henschen-Edman A., Aswad D. W., Stallcup M. R., Laird-Offringa I. A. Lipopolysaccharide-induced methylation of HuR, an mRNA-stabilizing protein, by CARM1. J. Biol. Chem. 2002;277:44623–44630. doi: 10.1074/jbc.M206187200. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Hanaoka K., Hayasaka M., Esumi E., Li S., Nonaka I. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Patapoutian A., Yoon J. K., Miner J. H., Wang S., Stark K., Wold B. Disruption of the mouse MRF4 gene identifies multiple waves of myogenesis in the myotome. Development. 1995;121:3347–3358. doi: 10.1242/dev.121.10.3347. [DOI] [PubMed] [Google Scholar]
- Radzioch D., Clayton M., Varesio L. Interferon-alpha, -beta, and -gamma augment the levels of rRNA precursors in peritoneal macrophages but not in macrophage cell lines and fibroblasts. J. Immunol. 1987;139:805–812. [PubMed] [Google Scholar]
- Rawls A., Morris J. H., Rudnicki M., Braun T., Arnold H. H., Klein W. H., Olson E. N. Myogenin's functions do not overlap with those of MyoD or Myf-5 during mouse embryogenesis. Dev. Biol. 1995;172:37–50. doi: 10.1006/dbio.1995.0004. [DOI] [PubMed] [Google Scholar]
- Rebane A., Aab A., Steitz J. A. Transportins 1 and 2 are redundant nuclear import factors for hnRNP A1 and HuR. RNA. 2004;10:590–599. doi: 10.1261/rna.5224304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin L. A., Rudnicki M. A. The molecular regulation of myogenesis. Clin. Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- van der Giessen K., Di-Marco S., Clair E., Gallouzi I. E. RNAi-mediated HuR depletion leads to the inhibition of muscle cell differentiation. J. Biol. Chem. 2003;278:47119–47128. doi: 10.1074/jbc.M308889200. [DOI] [PubMed] [Google Scholar]
- Xu Y. Z., Di Marco S., Gallouzi I., Rola-Pleszczynski M., Radzioch D. RNA-binding protein HuR is required for stabilization of SLC11A1 mRNA and SLC11A1 protein expression. Mol. Cell. Biol. 2005;25:8139–8149. doi: 10.1128/MCB.25.18.8139-8149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. K., Olson E. N., Arnold H. H., Wold B. J. Different MRF4 knockout alleles differentially disrupt Myf-5 expression: cis-regulatory interactions at the MRF4/Myf-5 locus. Dev. Biol. 1997;188:349–362. doi: 10.1006/dbio.1997.8670. [DOI] [PubMed] [Google Scholar]
- Zhang W., Behringer R. R., Olson E. N. Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev. 1995;9:1388–1399. doi: 10.1101/gad.9.11.1388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.