Abstract
Neural stem and progenitor cells typically exhibit a density-dependent survival and expansion, such that critical densities are required below which clonogenic progenitors are lost. This suggests that short-range autocrine factors may be critical for progenitor cell maintenance. We report here that purines drive the expansion of ventricular zone neural stem and progenitor cells, and that purine receptor activation is required for progenitor cells to be maintained as such. Neural progenitors expressed P2Y purinergic receptors and mobilized intracellular calcium in response to agonist. Receptor antagonists suppressed proliferation and permitted differentiation into neurons and glia in vitro, while subsequent removal of purinergic inhibition restored progenitor cell expansion. Real-time bioluminescence imaging of extracellular ATP revealed that the source of extracellular nucleotides are the progenitor cells themselves, which appear to release ATP in episodic burst events. Enzyme histochemistry of the adult rat brain for ectonucleotidase activity revealed that NTDPase, which acts to degrade active ATP and thereby clears it from areas of active purinergic transmission, was selectively localized to the subventricular zone and the dentate gyrus, regions in which neuronal differentiation proceeds from the progenitor cell pool. These data suggest that purine nucleotides act as proliferation signals for neural progenitor cells, and thereby serve as negative regulators of terminal neuronal differentiation. As a result, progenitor cell-derived neurogenesis is thus associated with regions of both active purinergic signaling and modulation thereof.
Keywords: ATP, neurogenesis, ventricular zone, precursor cells
Neural stem cells are self-renewing multipotential progenitor cells, whose daughter cells can differentiate into both neurons and glia (Gage, 2000; Luskin, 1993) (McKay, 1997; McKay, 1989) (Weiss et al., 1996) (Ourednik et al., 1999). Neural stem cells replicate themselves by symmetric division and clonal expansion, yet no humoral agents have yet been defined that specifically support these self-renewing divisions. Mitogens, including EGF and FGF, support the proliferation and expansion of neural progenitor cells, but cofactors appear to be required for their low density culture (Taupin et al., 2000). Neural stem cells expand as free-floating clusters in suspension culture, designated neurospheres by Weiss and colleagues, following their observation that substrate attachment impaired the capacity of neural stem cells for self-renewal (Morshead et al., 1994) (Reynolds et al., 1992) (Reynolds and Weiss, 1992).
Neural stem cells typically differentiate into both transit-amplifying committed progenitors and their neuronal and glial progeny when raised in monolayer cultures. Their need for close contact with one another may indicate that a short-range autocrine/paracrine signaling mechanism is required for the sustained proliferation of both neural stem and progenitor cells. The close-acting wnt/frizzled pathway has been implicated in neuronal mitogenesis, but not in neural progenitor cell self-renewal or expansion (Aubert et al., 2002; Lie et al., 2005). Glycosylated cystatin C (CCg) has been proposed as another locally acting agent, which appears to act as a co-factor to potentiate FGF2-activated progenitor cell division, permitting FGF-dependent expansion to operate at very low cell densities (Taupin et al., 2000). However, although CCg appears to be a promising autocrine/paracrine agent promoting progenitor cell expansion, it acts as a co-factor, and does not operate alone as a self-renewal factor.
On the basis of these observations, we asked what signaling systems might be employed by neural stem and progenitor cells to mediate their expansion. One highly conserved short-range signaling pathway, linked to the regulation of the cell cycle in eukaryotic cells, is represented by the P2Y family of purinergic receptors. Purine nucleotides, including ATP and ADP in particular, are endogenous metabolites that can also act as extracellular transmitters, acting through both the P2X and P2Y classes of ionotropic and metabotropic G protein-coupled receptors, respectively. Purinergic signaling has previously been implicated in the proliferation of a variety of undifferentiated cell phenotypes (Burnstock, 2002a; Burnstock, 2002b) (Sanches et al., 2002) (Wang et al., 1992), most notably tumor cells (Dixon et al., 1997) (Takano et al., 1994). In the nervous system, ATP has been identified as a mitogen for v-myc immortalized neural progenitor cells (Ryu et al., 2003), and ATP-mediated purinergic signaling through the P2Y1 receptor has been associated with developmental neurogenesis (Weissman et al., 2004). In addition, purines can signal through the Ras/Raf/MEK/MAPK pathway (Tu et al., 2000), indicating the potential for significant cross-talk with FGF, EGF/TGFα, PDGF and erbB-driven neural mitogenic pathways, among others.
Kriegstein and colleagues recently showed that P2Y1 receptor activation accompanied radial cell-derived neurogenesis in the developing rat forebrain and that suramin, a P2Y receptor antagonist, decreased proliferation (Weissman et al., 2004). We have now extended these observations by showing that: 1) the neural progenitor cells themselves as the source of local ATP; 2) ATP is made locally available in sporadic bursts rather than tonic release; 3) neuronal differentiation is accompanied by a marked down-regulation of purinergic signaling, evident by both a decrease in ATP release, and by loss of functional P2Y receptors; 4) serum, which potentiates terminal neuronal differentiation in a variety of systems (Goldman et al., 1992), potently inhibited purinergic signaling, and that; 5) neural progenitor cells are competent to differentiate into all neural phenotypes after reversal of their expansion by purinergic receptor antagonists. The latter observation in particular suggests that purines may act as self-renewal factors for neural stem cells. In accord with this possibility, we also noted that in vivo, purinergic regulation of neural progenitor cell expansion was manifested by the relative restriction of NTDPase activity to regions of active neurogenesis, in both the fetal ventricular zone, as well as in the highly neurogenic striatal wall and rostral migratory stream of the adult brain. Together, these observations indicate that purines act to support the self-renewing expansion of neural stem and progenitor cells, and suggest that the local modulation of extracellular purine availability may regulate neurogenesis in both fetal and adult mammalian forebrain.
MATERIAL AND METHODS
Neuronal cultures and neurospheres
Neuronal cultures were prepared from E16 mice and maintained as earlier described (Nedergaard, 1994). Cytosine arabinoside (Ara-C; 20 μM) was added at 48 hr to eliminate proliferating cells. Neural progenitor cells were isolated from E13 mice as previously described (Morshead et al., 1994) (Reynolds and Weiss, 1992). The forebrains were collected in a Ca/Mg-free Hank’s buffered saline solution (HBSS) and dissociated in 0.25% trypsin. The cells were resuspended at 4 × 106 cells/ml in DMEM/F12/N2 containing 10 ng/ml bFGF and 10 ng/ml EGF (Sigma, St. Louis, MO). The cells were plated at 4 ml/dish into 100 mm suspension culture plates and incubated at 37°C in 5% CO2, for at least a week to permit neurosphere generation The cells were then serially dissociated and repassaged at 4 × 106 cells/ml, 2–3 times before use.
Bioluminescence and calcium imaging
ATP release from living cells were dynamically imaged by chemiluminescence as described in (Arcuino et al., 2002). In order to normalize for the potential effect of serum on ATP release, we apportioned neurospheres into wells containing ascending concentrations of fetal bovine serum. These matched groups were raised in media containing 0, 0.1, 1, or 10% serum overnight, before the bioluminescence imaging or ATP content measurement. Neurospheres from each group were then spun and resuspended in Ringer’s solution and mounted in a temperature controlled Leiden chamber. Luciferase (0.132 mg/ml) and luciferin (0.332 mg/ml) was added to the Ringer solution. After obtaining a baseline recording of 5 minutes duration, the cultures were stimulated by adding an equal volume of Ca2+-free Ringer solution containing 100 μM UTP. Light production from the luciferin –luciferase reaction was imaged by a liquid nitrogen-cooled CCD camera (VersArray 1300B, Princeton Instruments), using a 20x oil lens (N.A. 0.8, Olympus), 8 × 8 binning, and two seconds integration. ATP content in samples collected from cultures grown in 24-well tissue culture plates was measured using a Victor2 plate reader (Wallac) (Cotrina et al., 1998a) and normalized to the protein content (BioRad) or to the cell number. A minimum of 8 independent experiments was evaluated (n ≥ 8, most >20). When drugs or serum were used, the standards were adjusted to containing an equivalent amount.
Immunocytochemistry, enzyme histochemistry, and cell cycle kinetics
Cultures were stained for nestin (monoclonal clone Rat 401, IgG1, or rabbit antiserum, Chemicon, Temecula, CA; 1:2000), neuronal class III β-tubulin (monoclonal clone TuJ1, IgG2a, Covance, Philadelphia, PA; 1:500), MAP-2 (2a+2b) (monoclonal clone AP-20, ascites fluid, Sigma, St. Louis, MO; 1:500), Neuronal protein HuC/HuD (monoclonal 16A11, Molecular Probes, Eugene, OR; 15 μg/ml), O4 (monoclonal IgM supernatant; O4 hybridoma a gift of Drs. S. Pfeiffer and R. Bansal, U. CT., Farmington, CT; 1:100), or GFAP (mouse clone GA5, or rabbit antiserum, Sigma; 1:500,), purinergic receptors P2Y1, P2Y2, P2Y4 (polyclonal, Alomone Labs, Jerusalem, Israel, 1:200), p27 (polyclonal, Chemicon, 1:2000), cyclins D1 and E (polyclonal, H-295 and M-20, respectively, Santa Cruz Biotechnology, Santa Cruz, CA; 1:100), Secondary antibodies were FITC-conjugated goat anti-mouse IgM (μ-chain specific, Sigma; 1:500), Cy3-conjugated goat-anti-rabbit IgG (H+L) (1:200), Cy5-conjugated Goat-anti-mouse IgG (H+L) (both from Jackson ImmunoResearch, West Grove, PA;1:100). Nuclei were counter-stained with Sytox Green or propidium iodide (Molecular Probes). P27, cyclin D and cyclin E FACS analyses were performed on an EPICS ELITE ESP flow cytometer/cell sorter (Beckman Coulter) as described (Deptala et al., 1999). For the analysis of ectonucleotidase activity (Braun et al., 2000b), cryosections from 4% paraformaldehyde-perfused rat brain were preincubated in a medium containing Tris-maleate buffer (50mM, pH7.4), sucrose (0.25M), CaCl2 (2mM) for 45 min at room temperature. Then tissue sections were incubated in substrate solution with 1 mM of substrate ATP (Sigma), Tris-maleate (50mM, pH7.4), MnCl2 (5mM), CaCl2 (2mM), Pb(NO3)2 (2mM), sucrose (0.25M), plus 1 mM levamisole (inhibitor of alkaline phosphatase; Sigma), 1mM ouabain (Na+, K+-ATPase inhibitor; Sigma), 50□M α,β-methylene-ADP (5′-nucleotidase inhibitor; Fluka) for 50 min at room temperature. Incubation was followed by three rinses in distilled water. Then samples were stained by incubating sections in solution of (NH4)2S (1% v/v). The same protocol was applied for the substrates ADP and IDP. In control experiments, substrate was omitted.
Cell proliferation
For the BrdU incorporation assay, the cultures were incubated for 4 hrs in BrdU (Sigma, 10 μg/ml, 4 hrs), fixed and stained with rat monoclonal anti-BrdU (MAS250C, Harlan Sera-lab, Loughborough, UK; 1:200) and Cy3-conjugated goat anti-rat antibodies (Jackson ImmunoResearch, 1:300). Nuclei were visualized by Sytox (Molecular Probes, Inc.) and the mitotic index calculated as the ratio of BrdU/Sytox positive nuclei. Limiting dilution analysis was performed as previously described (Keyoung et al., 2001) (Uchida et al., 2000).
RESULTS
Purines signaling is restricted to primary neural precursor cells
To test the idea that purinergic signaling is exhibited by undifferentiated neural precursor cells - by which we do not distinguish between neural stem cells and transit-amplifying neural progenitor cells – but not by their differentiated daughters, we first examined Ca2+ responses to ATP exhibited by both neural progenitor cells and differentiated neurons. Mouse cortical neurons raised in highly enriched neuronal cultures were loaded with fluo-4/am and exposed to either ATP (100 μM) or K+ (60 mM). Transient increments in Ca2+ were triggered by ATP, but not by high K+, in cultures younger than 2 days in vitro (DIV) (fig. 1A). The responses to ATP fell by day 3, and were absent 9 days after plating. In contrast, the maturing neurons gained sensitivity to K+ by day 3, and exhibited a many-fold increase in cytosolic Ca2+ in response to high mM K+ from day 9 (fig. 1B). Immunostaining revealed that the P2Y1, P2Y2, and P2Y4, and purinergic receptors were abundantly expressed by nestin+ progenitor cells, but that the expression of each fell with neuronal maturation (fig. 1C). By 30 hours after plating, MAP2-defined neurons expressed substantially less P2Y receptor-immunoreactivity than did neighboring, MAP2− cells (fig. 1C). By 5 days, P2Y-immunoreactivity was restricted to GFAP+ astrocytes.
Figure 1. Neuronal differentiation is accompanied with loss of purinergic signaling.
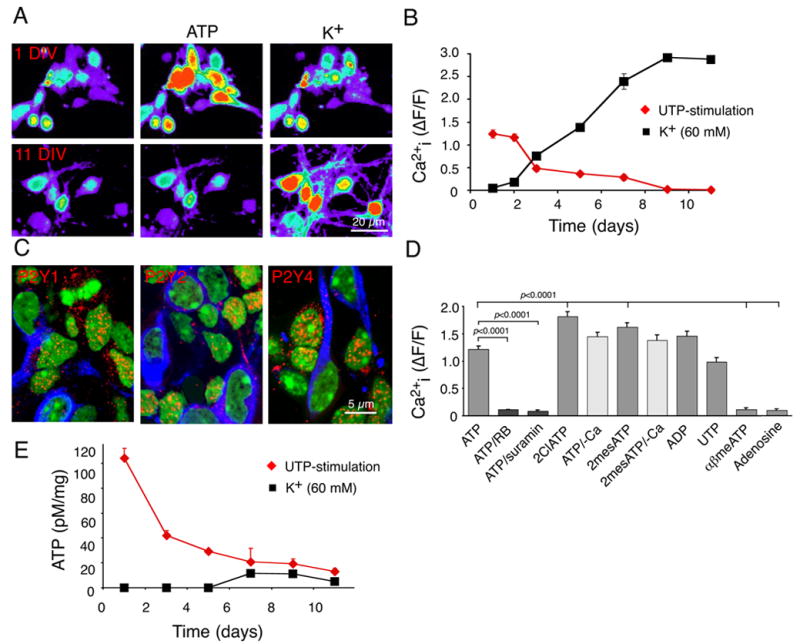
(A) Ca2+ responses of primary cortical neurons at 1 and 11 days in vitro (DIV) to ATP (100 μM) and K+ (60 mM). The cultures were loaded with the calcium indicator fluo-4/am (45 min, 4.6 μM). (B) Ca2+ responses to ATP and K+ as a function of DIV. means ± SEM (C) Expression of P2Y1, P2Y2, and P2Y4 (red) at 1 DIV. Expression of P2 receptors were lower in MAP2-positive neurons (blue) than in MAP2-negative cells. (D) Ca2+ responses to ATP and ATP analogs. The potency by which the ATP agonists mobilized intracellular Ca2+ stores is compatible with expression of functional P2Y1 (ADP), P2Y2 and P2Y4 (UTP) receptors. (E) ATP release in response to stimulation by UTP (100 μM) and high K+ (60 mM) as a function of DIV. The pseudocolor scale in A is similar to that in fig. 2B. Neurons were prepared from E16 mice pups, whereas neurospheres were prepared from E13 mice pups.
The ATP-induced increase in Ca2+i in 1–2 day cultures was concentration-dependent, with an IC50 of 8.8 ± 1.5 μM. ATP-induced Ca2+i increments were inhibited by both suramin (100 μM) and reactive blue (RB; 30 μM), two mechanistically-unrelated inhibitors of purinergic signaling (fig. 1D). A variety of purine receptor agonists, that included ATP, ADP, UTP, 2-Cl-ATP, γSATP, and 2-MeSATP, increased Ca2+i with roughly equal potency. Removal of extracellular Ca2+i did not significantly decrease the response to ATP or 2-MesATP. Taken together, these results suggest that ATP primarily mobilized intracellular Ca2+ stores, rather than opening Ca2+ permeable channels in neural progenitor cells. Consistent with this notion, αβATP - a P2X specific agonist, failed to evoke Ca2+ responses, while oxidized ATP (300 μM, 1 hr, a P2X7 antagonist) did not significantly reduce ATP-evoked Ca2+ responses (p = 0.4, Student’s t test).
Purines are released as autocrine factors, and signal through P2Y receptors
We next tested the postulate that neural progenitor cells might secrete purine nucleotides, thereby allowing nucleotides to promote proliferation through an autocrine/paracrine signaling pathway. We found that purine receptor activation by UTP (a P2Y agonist, with a potency similar to that of ATP) evoked robust ATP release by neuroepithelial cells during their first 2 days in vitro, but not in more differentiated cultures (fig. 1E) (Cotrina et al., 1998a) (Cotrina et al., 1998b). Importantly, high potassium failed to elicit ATP release from mature neurons, despite triggering robust increases in cytosolic calcium. Thus, neurons lose their ability to release ATP in response to elevations of Ca2+i, concomitant with their down-regulation of P2Y receptors (fig. 1E). In contrast, if the same cells were cultured as free floating neurospheres, the uncommitted nestin+ progenitor cells continued to express P2Y receptor-immunoreactivity (fig. 2A), and to respond to purine agonists (ATP) with increments in cytosolic Ca2+ after repetitive passage (162% ± 15%) (fig. 2B, supplemental movie). Spontaneous increases in Ca2+ were infrequent (0.0048 ± 0.0035 cell/sec). An analysis of spontaneous Ca2+ increases showed that the relative mean increase in Ca2+ was 193 ± 15%, lasted for 21 ± 2 sec. Neurospheres promptly responded to all purinergic agonists tested. Ca2+ increases in response to UTP, ADP, 2ClATP, 2meSATP (100 μM) did not differ significantly from ATP induced Ca2+ increases (p = 0.78, ANOVA). Following exposure to purinergic agonists, the spontaneous Ca2+ increases dispappeared. In contrast, to cultured neurons, the neurosphere-resident cells failed to exhibit any increase in Ca2+ when exposed to 60 mM K+ (Fig. 1). Of note, purinergic receptor antagonists, including suramin (300 μM), reactive blue (40 μM), and PPADS (50 μM), all failed to alter baseline Ca2+ signal following a 30–60 min incubation period (ANOVA, p = 0.66).
Figure 2. Persistence of purinergic signaling in neurospheres.
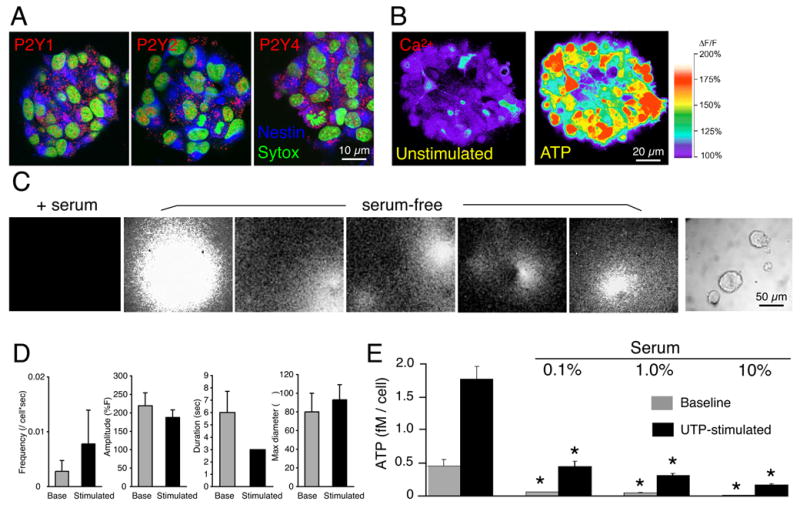
(A) Immunostaining against P2Y1, P2Y2, and P2Y4 receptors in neural spheres (6 weeks/passage 3. (B) ATP (100 μM) triggered increases in cytosolic Ca2+ in a neural sphere (3rd passage). Spheres were loaded with fluo-4/am (4.6 μM, 30 min) and imaged by confocal microscopy. (C) Bioluminescence imaging of ATP release from neural spheres. Repeated ATP burst release event from spheres maintained in serum-free medium after stimulation, but not from the spheres in cultured in 1% serum overnight (+serum). Representative bioluminescence recordings from several different cultures are shown. Right panel depicts one of the sphere cultures utilized for imaging in phase contrast. (D) Histograms summarizing frequency, amplitude, and duration of ATP burst events (6 weeks, passage 3; n = 180–470 cells, 3 – 6 experiments) (E) Serum reduced in a dose-dependent manner unstimulated (baseline) and stimulated ATP release (100 μM UTP). ATP concentrations were measured in samples collected before and after stimulation. *P < 0.01, one-way ANOVA, Bonferroni posthoc test. Neurospheres were prepared from E13 mice pups
Purinergic receptors are characterized by rapid and sustained desensitization in response to agonist exposure, and transient repeated episodes of agonist exposures are more efficient than chronic stimulation (Cotrina et al., 1998a). To address the question of the source and mechanism of ATP release, we next visualized the pattern of ATP release from neurospheres using bioluminescence imaging. Specifically, we added a mixture containing luciferase and its substrate, luciferin, to the culture medium. By this approach, ATP release can be monitored by light emissions resulting from the ATP-triggered luciferase breakdown of luciferin, both at the single-cell level and in real time, using a liquid nitrogen-cooled CCD camera (fig. 2C, supplemental movies) (Arcuino et al., 2002). At baseline, the majority of the neural spheres exhibited infrequent point-source bursts of light emission. The frequency of ATP burst releases showed a non-significant increase (p = 0.419, t-test) after UTP stimulation. The ATP burst releases were variable in their duration and extent of spatial expansion, but were abrupt in onset and spherical in their spread, likely reflecting ATP diffusion from a point-source (Arcuino et al., 2002). No differences in amplitude, duration, or maximal diameter were noted before and after stimulation (fig. 2D).
Differentiation was attended by a loss of purine release and reception
Serum and serum-borne factors have been shown to be both anti-mitotic and pro-differentiative for neural progenitor cells. We found that serum was a potent inhibitor of both ATP release and purinergic signaling. Bioluminescence imaging revealed a virtually complete suppression of ATP burst events in as little as 1% serum (n= 34 spheres, 0 ATP release events observed), whereas matched serum-free cultures exhibited a total of 156 ATP bursts (n=44 spheres; p<0.001 by χ2 analysis) (fig. 2E). Accordingly, analysis of ATP in samples of the medium revealed that 1% serum addition was associated with a >80% decline in the extracellular ATP concentration, and a >90% fall in UTP-stimulated ATP release. Furthermore, the serum-associated suppression of purine-induced ATP release was an inverse function of the serum concentration (fig. 2E). Thus, serum addition led to both an abolition of ATP release and a down-regulation of P2Y receptors and agonist-evoked responses, that attended cellular differentiation. These observations suggest that the progenitor cells themselves are the source of ATP, which through an autocrine mechanism triggers P2Y receptor-mediated mobilization of intracellular calcium.
Purines are mitogens for neural precursors, and signal through P2YR
Purinergic signaling has long-term effects on proliferation of numerous cell types, including rapidly replicating transformed cell lines (Burnstock, 2002b) (Michoud et al., 2002) (Sauer et al., 2002) (Tu et al., 2000). To better define the mitogenic effects of P2Y on neural progenitor cells, we next compared the effects of ATP, its analogs and its dephosphorylated metabolites, on precursor cell division. To this end, we quantified bromodeoxyuridine (BrdU) incorporation by neural progenitor cells after 4 hrs incubation in each treatment. ATP increased proliferation in a dose-dependent manner, peaking at 100 μM. Several ATP analogs, including ADP, ATPγS, 2Cl-ATP, and UTP, had comparable effects, with the exception of the P2X agonist αβATP (Fig. 3A, left panel). The mitogenic action of nucleotides was not a result of hydrolysis of ATP to adenosine, since ATPγS is non-degradable, and neither adenosine nor NECA (a broad-spectrum A1 and A2 adenosine agonist) stimulated BrdU incorporation. A combination of the adenosine receptor antagonists DPCPX and MRS 1191 was also without effect, suggesting that P1 adenosine receptors do not contribute to ATP-triggered mitosis (Fig. 3A, middle panel).
Figure 3. Purinergic signaling sustained the proliferation of neural progenitors.
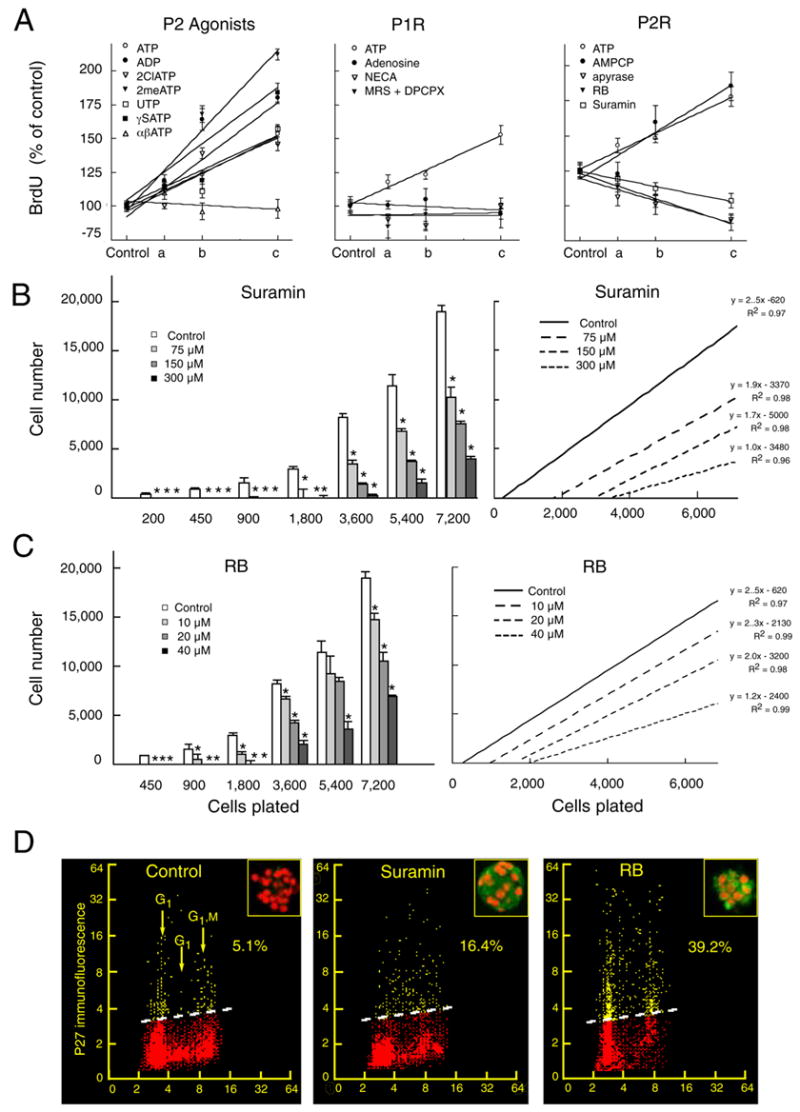
(A) The mitotic index of neural progenitors determined by the BrdU incorporation assay. The effect of ATP, ADP, 2ClATP, 2meSATP, UTP, γSATP, or αβATP upon BrdU incorporation relative to vehicle-treated controls was quantified at; 0 μM (control), 25 μM (a), 50 μM (b), and 100 μM (c) (left panel). Effect of ATP, adenosine, and NECA at; 0 μM (control), 25 μM (a), 50 μM (b), and 100 μM (c). A combination of the adenosine receptor antagonists DPCPX and MRS-1191 were tested at increasing concentration; 0 μM + 0 μM (control), 100 μM + 5 μM (a), 200 μM + 10 μM, and 400 μM + 20 μM (middle panel, same y-axis as left panel). Effect on BrdU incorporation of ATP or the ectonuclease inhibitor AMPCP; at 0 μM (control), 25 μM (a), 50 μM (b), and 100 μM (c). Apyrase was tested at: 0 U/ml (control), 10 U/ml (a), 20 U/ml (b), 40 U/ml (c). RB at; 0 μM (control), 10 μM (a), 20 μM (b), 40 μM (c). Suramin at; 0 μM (control), 75 μM (a), 150 μM (b), 300 μM (c) Lines represents 1-order linear regression. Regression coefficients were in the range of 0.89 to 0.99 (right panel). (B–C) Suramin and RB increased the minimal plating density required for survival of neural progenitors. At low plating densities, cells cultured in presence of suramin or RB died by 5 days in vitro *P < 0.01, one-way ANOVA, Bonferroni posthoc test. Right panels display 1-order regression analysis of the data in the left panel. (D) FAC analysis demonstrates an upregulation of the mitotic repressor, P27 in neural progenitor cells exposed to suramin (300 μM) and RB (40 μM). Bivariate distributions (scattergrams) representing DNA content (cell cycle distribution) versus expression of p27 in individual progenitor cells. The percent of p27 positive cells (above the threshold lines) was quantified based on the level of fluorescence of control cells stained with the secondary antibody only (isotypic control). Inserts Neural spheres immunostained against p27 (green). Nuclei are labeled with propidium iodide (red). Neurospheres were prepared from E13 mice pups.
In accord with the role of ATP and ADP-dependent P2Y signaling, the ectonucleotidase inhibitor α,β-methyl adenosine diphosphate (AMPCP), which blocks the breakdown of ATP and ADP to AMP, potently enhanced the expansion of neural progenitors. In contrast, the ATPase apyrase, which hydrolyses ATP and ADP to AMP, inhibited BrdU incorporation. In addition, the two P2Y receptor antagonists, suramin and Reactive Blue (RB), inhibited the mitotic index in a dose-dependent manner (Fig. 3A, right panel).
Purinergic blockade increased the cell densities required for neurosphere propagation
We next assessed the role of P2Y signaling in progenitor cell proliferation, by assessing both the minimum cell density requirement for neurosphere generation, and the absolute expansion thereof, during concurrent sustained exposure to both agonists and antagonists of purinergic signaling. To this end, neurospheres were dissociated to low density cultures and limiting dilution analysis performed, in the presence or absence of either suramin or RB, as broad-spectrum inhibitors of P2Y receptors. Test agents were added once daily to third passage neurospheres, and vehicle to control cultures, for 5 days of continuous exposure. Both suramin and RB increased the minimum density at which the cells could remain viable and expand (Fig. 3B–C). Thus, purinergic receptor activation was required not only for continued proliferation of neural progenitors, but also for survival at low plating densities. However, we did not find the converse to be true: ATP agonists did not lower the cell density at which progenitor cell survival could be achieved. We found that the addition of the purine agonists ATP or ATPγS (5–50 μM) had no effect on cell number, when assessed after 5 days of incubation; increasing the concentration of ATP or ATPγS beyond 50 μM resulted in cell death. These data suggested that purinergic receptor activation potentiates the division of neural progenitor cells, but that sustained purinergic agonism alone may be insufficient to support progenitor cell survival and proliferation.
The fleeting nature of purine-associated mitogenesis may have been a product of receptor desensitization during sustained agonist exposure. Indeed, the apparent transience of ATP-dependent progenitor cell mitogenesis may not reflect normal physiology, in which ATP is released not tonically, but rather in transient burst events. In vivo, purinergic signaling might regulate the expansion of neural progenitors in a manner dependent upon the integrated frequency and amplitude of ATP burst events, rather than by sustained changes in interstitial ATP levels. Purinergic receptor agonists in vitro may thereby fail to lower the cell numbers required for neurosphere propagation, since sustained agonist exposure, and its attendant receptor desensitization, may not replicate the transient local increases of ATP experienced by progenitors in vivo.
Purinergic inhibition decreased the mitotic index of neural progenitor cells
Flow cytometry confirmed that the purinergic inhibitors decreased the fraction of cells in S phase: 16.4% ± 1.8% of cells acutely dissociated from untreated control neurospheres were in S-phase, compared to 5.7 ± 0.3% and 8.4 ± 2.3 in RB and suramin treated cultures, respectively (p < 0.001) (Deptala et al., 1999). The inhibition of proliferation afforded by suramin and RB was reversible. The percentage of cells in S-phase recovered by 50% at 8 h and by 80% at 24 hr after wash-out of the drugs. Suramin-treated cultures also exhibited an increase in the tumor suppressor P27, a strong negative regulator of cell division. P27 expression rose more strongly in RB- than suramin-treated cultures, pare passu with the stronger inhibition of cell proliferation afforded by RB (Fig. 3D).
Purinergic blockade did not affect phenotypic differentiation
The lineage potential of progenitors whose division was suppressed by suramin or RB was examined by clonal expansion. In brief, neural spheres rendered mitotically quiescent by 5 day exposures to suramin and RB were dissociated and plated at low density in fresh medium without inhibitors. Seven days later, the cultures were fixed and immunostained for a panel of linage associated markers. Immediately after plating, both sets of cultures strongly expressed nestin, a marker of immature precursors. After 7 d in cultures a significant number of the cells had differentiated into neurons, astrocytes, and oligodendrocytes (<1–3%) (fig. 4A). Importantly, neither suramin nor RB restricted the lineage potential of exposed progenitor cells. After re-expansion, the antagonist-treated cultures contained neurons, astrocytes, and oligodendrocytes, and in the same relative proportions as their untreated controls (fig. 4B). These results indicated that the suppression of progenitor cell expansion induced by RB and suramin did not influence the differentiation paths available to the progeny of those cells.
Figure 4. Purinergic blockade initiated the differentiation of all major neural lineages.
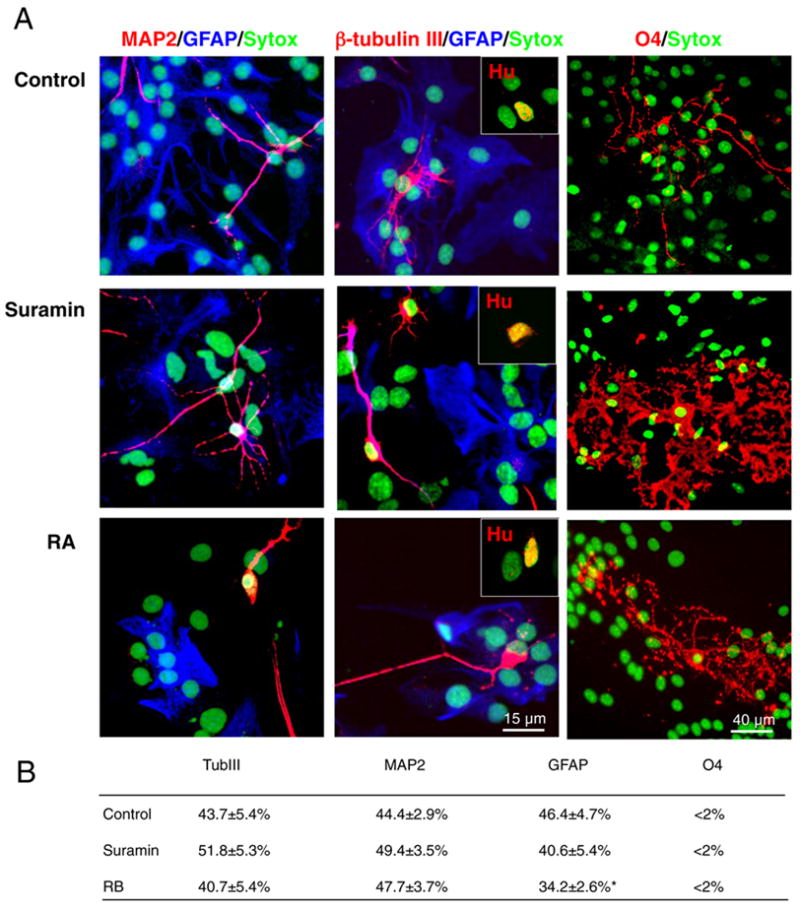
Immunostaining revealed that all major lineages were generated from neural progenitor cells upon suramin-mediated P2Y blockade. Neurospheres were exposed to suramin (100 μM) or RB (40 μM) for 5 to 7 days and then differentiated in 1% serum after plating on laminin. (A) Neurons (MAP2, β-tubulin III, Hu), astrocytes (GFAP) and oligodendrocytes (O4) were co-generated following cell cycle exit. (Nuclei stained with Sytox, green). (B) Table summarizing the expression of neuronal and glial markers after 7 days of differentiation in presence of 1% serum. *P < 0.01, one-way ANOVA, Bonferroni posthoc test. Neurospheres were prepared from E13 mice pups.
Ectonucleotidase activity was associated with undifferentiated cells
Ectonucleotidases are extracellular ATP hydrolyzing enzymes, that modulate purinergic signaling by rapidly degrading ATP. NTDPase-mediated dephosphorylation of ATP provides AMP, a poor agonist for P2Y receptors. As such, NTDPase may act as a brake on local P2Y signaling (Braun et al., 2003). On this basis, we used enzyme histochemical analysis to assess ectonucleotidase activity, both in vitro and in vivo. In culture, neurospheres exhibited intense and uniform staining when using either ATP or ADP as a substrate for lead-deposition ectonucleotidase histochemistry. Plating of the neurospheres or exposure to serum each resulted in rapid and sharp decreases in staining intensity (data not shown). Thus, uncommitted progenitor cells were characterized not only by a high level of P2Y receptor expression and spontaneous ATP release, but also by high level of endogenous ectonucleotidase activity. During neural differentiation, P2Y receptor expression fell, both ATP release and ATP-triggered cytosolic Ca2+ mobilization were attenuated, and these events were attended by a fall in ectonucleotidase activity and consequent drop in available agonist.
P2Y receptors and ectonucleotidase activity localized to neurogenic regions of brain
To assess the involvement of ATP/P2Y receptor signaling in neural differentiation in vivo, we next analyzed the expression of P2Y receptors and ectonucleotidase activity in both fetal (E14) and adult rat brain (Fig. 5A). In the fetal brain, the receptor density appeared evenly distributed across the developing cortex, whereas a relative higher abundance of P2Y receptor expression was evident in the subventricular zone of adult animals. Similar pattern of expression of P2Y1 and P2Y4 receptors were observed (data not shown).
Figure 5. ATP ectonucleotidase activity localizes sharply to neurogenic regions of the brain.

(A) P2Y receptor expression extended throughout the telencephalic ventricular zone of embryonic brain (E14). P2Y expression was more sharply localized to ependymal and subependymal layer of the adult ventricular wall (P51). (B) ATP ectonucleotidase activity (left) in the adult brain was localized to the striatal ventricular wall, extending dorsolaterally to the take-off of the rostral migratory stream (inset). A no-ATP control for the specificity of the ectonucleotidase histochemistry, right). Lesser degrees of enzyme activity were also noted in the callosal wall. (C) The subgranular zone of the dentate gyrus also exhibited prominent ectonucleotidase activity. The striatal ventricular wall, RMS and dentate are the only persistently neurogenic regions of the adult brain, and were the only regions to exhibit high levels of ectonucleotidase activity. (D) Comparison of ATP ectonucleotidase activity in the dentate (subgranular zone (SGZ), the molecular layer, and the granular zone), as well as the ventricular zone, rostral stream, stratum radiatum, and corpus callosum. Enzyme activity was quantified on representative bright field images in 3 independent experiments. Insert Comparison of relative enzyme activity in non-neurogenic areas (including the molecular and granular zone of the dentate, as well as the stratum radiatum and corpus callosum) and neurogenic zone (SGZ, ventricular zone, and rostral stream). Neurogenic zones exhibited a substantial higher ATP ectonucleotidase activity than non-neurogenic zones. *; p < 0.0001, t-test.
Enzyme histochemical analysis was next utilized to map ectonucleotidase activity in brain sections (Fig. 5B–C). The enzyme reaction was performed on cryostat sections incubated in either ATP or ADP as substrate. The analysis revealed a striking pattern of high enzyme activity corresponding to neurogenic areas in adult brain. In the ventricular wall, high enzyme activity was evident in the subependymal zone, especially along the striatal wall and its rostrolateral tip, from which arises the highly neurogenic rostral migratory stream. In addition, as previously been described, the subgranular zone (SGZ) of the hippocampus expressed high ectonucleotidase activity, with striking restriction to the region of dentate granule cell neurogenesis (Figs. 5B–D).
DISCUSSION
These experiments revealed that ATP acts as a mitogenic signal for neural stem and progenitor cells, and may do so in an autocrine fashion. We found that when maintained as mitotically-active neurospheres, neural progenitor cells exhibited both ATP release and purinergic receptor-activated calcium mobilization. Real-time bioluminescence imaging of ATP release revealed that the progenitor cells themselves were the source of ATP, which they released in brief burst events. External addition of ATP or its analogs increased the mitotic index and rate of neural progenitor cells, whereas P2Y antagonists suppressed both neurosphere expansion and the mitotic index of cells within those spheres. Both ATP release and purine-activated calcium responses were retained over several months of repetitive passage, as was the reversible suppression of neurosphere expansion by P2Y antagonists. Strikingly, both the mitotic competence and multilineage potential of the cultured neural progenitor cells were restored upon P2Y antagonist removal. Indeed, the relative fractions of neurons, astrocytes, and oligodendrocytes that developed after plating of neurospheres were essentially unchanged in controls versus antagonist treated cultures. This contrasted to the expansion associated with other described positive regulators of neural progenitor cell expansion, such as sonic hedgehog, which have typically been associated with a sustained loss of neuronal differentiation competence (Wechsler-Reya and Scott, 1999).
Kriegstein and colleagues (Weissman et al., 2003) demonstrated that calcium waves and attendant ATP release, with consequent P2Y1 receptor activation, accompanied radial cell-derived neurogenesis in cultured slices of the developing rat forebrain. This model permitted an assessment of BrdU incorporation for only 1 hour after ATP exposure, which was sufficient only to demonstrate accelerated DNA replication in the presence of ATP. In our present report, we noted that proliferation was indeed sustained for at least 5 days in the presence of ATP, indicating the competence of purinergic signaling in mediating progenitor cell proliferation and expansion. In addition, whereas Weissman et al identified primarily P2Y1-mediated purinergic transmission among radial cells, whose responses were thereby limited to ATP, we found that NSCs expressed P2Y-1, 2 and 4 receptors, and were hence sensitive to both ATP and UTP. These observations suggest a greater heterogeneity of purinergic signals, and hence of purine-mediated functions, in neural progenitor cells than revealed by slice culture. In this regard, real-time bioluminescence imaging of of ATP release by neurospheres revealed that neural progenitor cells were themselves the sources of local ATP. In particular, we noted that ATP was released by individual cells within each neurosphere in brief, evanescent bursts, that were exhibited by only a minor fraction of the cells, and through which ATP was effectively made available as a local and autocrine mitogen. This pattern of release was of especial interest since P2Y receptors can be desensitized for as long as 2 hrs after activation. Because purinergic receptors exhibit rapid desensitization to sustained agonist exposure, such brief bursts of transmitter release might then better stimulate proliferation than a more sustained stimulus, to which the cells might quickly become refractory (Burnstock, 2002a). As a result, single brief bursts of ATP followed by local receptor inactivation suggest a burst-suppression model by which local populations of neural progenitor cells might be effectively synchronized and brought under the mitotic direction of single cells pacing the time course of ATP release.
Several lines of work have provided evidence for the importance of transient changes in Ca2+. For example, Spitzer and co-workers showed that naturally occurring patterns of Ca2+ transients encoded neuronal differentiation (Spitzer et al., 2004). Following closure of the neural tube, spinal cord neurons exhibit repeated Ca2+ transients produced by Ca2+ dependent action potentials. Pharmacological manipulations showed that neuronal differentiation was altered when Ca2+ transients were eliminated by blocking Ca2+ influx. Conversely, re-imposing different frequency patterns of Ca2+ elevation were sufficient to promote neuronal differentiation, including normal neurotransmitter expression and channel maturation (Gu and Spitzer, 1995).
We have previously identified point-source bursts of ATP in cultured astrocytes, which exhibit a channel-mediated efflux of cytosolic ATP (Arcuino et al., 2002). This suggested a role for purinergic signaling in the functional maintenance of the astrocytic syncytium. In this regard, astrocytes have been implicated in the support of neurogenesis from progenitor cells in neurogenic regions of the adult brain (Lim et al., 2000) (Gage, 2000) (Song et al., 2002). As a result, the paracrine activation of neural progenitor cells by their astrocytic neighbors might then serve to maintain their undifferentiated self-renewal. As such, astrocytic ATP may be viewed as negatively regulating neurogenesis, by maintaining the mitotic activity of resident precursors, while suppressing their terminal neuronal or glial differentiation.
In accord with this model, both serum addition and substrate anchorage substantially reduced the ability of neural progenitors to release ATP (Fig. 2E), consistent with the differentiative effects of both serum and substrate on neural progenitor cells (Goldman et al., 1992) ((Reynolds and Weiss, 1992). Indeed, nucleotide-mediated signaling was rapidly lost during neuronal differentiation: Purinergic receptor expression was uniformly down-regulated early in the process of neuronal differentiation, and exposure to receptor agonists failed to mobilize cytosolic calcium in MAP-2+ neurons. Together, these observations suggest that neural progenitor cells both release ATP, and respond to it with an increase in proliferation. As such, ATP appears to act through both autocrine and paracrine routes to regulate the division and proliferation of neural progenitor cells. The high ectonucleotidase activity of cultured neurospheres may serve to modulate this process by regulating ATP and ADP bioavailability, thereby allowing neurogenesis to proceed despite the active purinergic signaling needed for expanding the progenitor cell pool antecedent to neuronal differentiation. Indeed, to the extent that NTDPase activity may act to remove local ATP and ADP and hence diminish local purinergic signaling, the expression of NTDPase by neural progenitor cells may be critical to their production of neurons. In this regard, neurospheres typically generate a fraction of neurons peripherally, pare passu with the concurrent central expansion of uncommitted neural progenitor cells. It would be fair to note, however, that our observation does not provide direct evidence for the importance of ATP burst release vs tonic release of ATP. Technical limitations prevented us from discriminating between the two pathways of ATP release. Nevertheless, our observations strongly suggest that the regulation of purinergic signaling may be critical to neural progenitor cell expansion.
ATP release and purinergic signaling may be required not only for developmental progenitor cell expansion and neurogenesis, but also to the persistent progenitor cells of the adult brain. The co-localization of both P2Y receptors and ectonucleotidase activity to regions of active mitotic progenitor cell expansion and neurogenesis in the adult brain (Braun et al., 2003; Shukla et al., 2005) is especially significant in this regard, given the apparent necessity of purinergic signaling to neural progenitor cell expansion in vitro. Just as neural progenitor cells appear to undergo ATP release as a means of both autocrine and paracrine purinergic activation of self-renewing divisions, their local production of NTDPase may serve as a brake on that process, clearing ATP and preventing uncontrolled expansion while establishing a permissive microenvironment for neuronal differentiation. By delimiting progenitor cell proliferation, the NTDPase-mediated clearance of ATP may thereby provide an important permissive condition for neurogenesis, and hence for the maintenance of local neurogenic niches. Indeed, the association of NTDPase activity with the capillary microvasculature (Braun et al., 2000a) suggests that purinergic signaling may contribute to the angiogenic support of neurogenesis in adult neurogenic niches (Cleaver and Melton, 2003; Louissaint et al., 2002; Palmer et al., 2000). As a result, progenitor cell-derived neurogenesis may require both active purinergic signaling and the negative regulation thereof.
Our data suggest that ATP burst-activated purinergic signaling may constitute a critical regulatory checkpoint for modulating the expansion and lineage commitment by neural progenitor cells. As such, its abrogation, through a suppression of either purine release or reception, may comprise a means of inhibiting undesired progenitor or progenitor cell expansion, as may be the case in neoplasias of the CNS (Burnstock, 2002a). In contrast, its stimulation may provide a means of expanding progenitor cell populations, so as to provide an expanded cellular substrate for strategies designed to induce neurogenesis from endogenous progenitor cell pools. On this basis, the pharmacological regulation of purinergic signaling may permit us as a means of modulating neurogenesis in both the fetal and adult brain.
Supplementary Material
Acknowledgments
Supported by NS38073 and NS41031 (MN), and NS33106, NS39559 and NS29813 (SG). We thank Neeta Roy, Abdellatif Benraiss, Qun Gao, and Jan Kunicki for advice and assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A. 2002;99:9840–5. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert J, Dunstan H, Chambers I, Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 2002;20:1240–5. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Mishra S, Robson S, Barth S, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003;17:1355–64. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Robson S, Enjyoji K, Guckelberger O, Hammer K, Di Virgilio F, Zimmermann H. Assignment of ectonucleoside triphosphate diphosphohydrolase-1/CD39 expresssion to microglia and vasculature of the brain. Eur J Neurosci. 2000a;12:4357–4366. [PubMed] [Google Scholar]
- Braun N, Sevigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, Di Virgilio F, Zimmermann H. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci. 2000b;12:4357–66. [PubMed] [Google Scholar]
- Burnstock G. Potential therapeutic targets in the rapidly expanding field of purinergic signalling. Clin Med. 2002a;2:45–53. doi: 10.7861/clinmedicine.2-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002b;22:364–73. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Melton D. Endothelial signaling during development. Nature Medicine. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- Cotrina M, Lin JH-L, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CCG, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci. 1998a;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998b;18:8794–804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deptala A, Li X, Bedner E, Cheng W, Traganos F, Darzynkiewicz Z. Differences in induction of p53, p21WAF1 and apoptosis in relation to cell cycle phase of MCF-7 cells treated with camptothecin. Int J Oncol. 1999;15:861–71. doi: 10.3892/ijo.15.5.861. [DOI] [PubMed] [Google Scholar]
- Dixon CJ, Bowler WB, Fleetwood P, Ginty AF, Gallagher JA, Carron JA. Extracellular nucleotides stimulate proliferation in MCF-7 breast cancer cells via P2-purinoceptors. Br J Cancer. 1997;75:34–9. doi: 10.1038/bjc.1997.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Zaremba A, Niedzwiecki D. In vitro neurogenesis by neuronal precursor cells derived from the adult songbird brain. Journal of Neuroscience. 1992;12:2532–41. doi: 10.1523/JNEUROSCI.12-07-02532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–7. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Keyoung HM, Roy N, Louissant A, Benraiss A, Mori Y, Okano H, Goldman SA. Specific identification, selection and extraction of neural stem cells from the fetal human brain. Nature Biotechnology. 2001;19:843–850. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- Lie D, Colamarino S, Song H, Desire L, Mira H, Consiglio A, Lein E, Jessberger S, Lansford H, Dearie A, Gage F. Wnt signaling regulates adult neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lim D, Tramontin A, Trevejo J, Herrera D, Garcia-Verdugo J, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- McKay RD. The origins of cellular diversity in the mammalian central nervous system. Cell. 1989;58:815–21. doi: 10.1016/0092-8674(89)90934-3. [DOI] [PubMed] [Google Scholar]
- Michoud MC, Napolitano G, Maghni K, Govindaraju V, Cogo A, Martin JG. Effects of extracellular triphosphate nucleotides and nucleosides on airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 2002;27:732–8. doi: 10.1165/rcmb.4768. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J, Park KI, Snyder EY. Neural stem cells -- a versatile tool for cell replacement and gene therapy in the central nervous system. Clin Genet. 1999;56:267–78. doi: 10.1034/j.1399-0004.1999.560403.x. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comparative Neurology. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–74. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLarnon JG, Kim SU. Adenosine triphosphate induces proliferation of human neural stem cells: Role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res. 2003;72:352–62. doi: 10.1002/jnr.10507. [DOI] [PubMed] [Google Scholar]
- Sanches G, de Alencar LS, Ventura AL. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Int J Dev Neurosci. 2002;20:21–7. doi: 10.1016/s0736-5748(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Sauer H, Stanelle R, Hescheler J, Wartenberg M. The DC electrical-field-induced Ca(2+) response and growth stimulation of multicellular tumor spheroids are mediated by ATP release and purinergic receptor stimulation. J Cell Sci. 2002;115:3265–73. doi: 10.1242/jcs.115.16.3265. [DOI] [PubMed] [Google Scholar]
- Shukla V, zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sevigny J, Robson S, Braun N. Functional expression of the ecto-ATPase NTPDase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J Neurosci Res. 2005;80:600–610. doi: 10.1002/jnr.20508. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–21. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Takano S, Gately S, Engelhard H, Tsanaclis AM, Brem S. Suramin inhibits glioma cell proliferation in vitro and in the brain. J Neurooncol. 1994;21:189–201. doi: 10.1007/BF01063768. [DOI] [PubMed] [Google Scholar]
- Taupin P, Ray J, Fischer W, Suhr S, Hakansson K, Grubb A, Gage F. FGF2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28:385–397. doi: 10.1016/s0896-6273(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Tu MT, Luo SF, Wang CC, Chien CS, Chiu CT, Lin CC, Yang CM. P2Y(2) receptor-mediated proliferation of C(6) glioma cells via activation of Ras/Raf/MEK/MAPK pathway. Br J Pharmacol. 2000;129:1481–9. doi: 10.1038/sj.bjp.0703182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck D, He D, Reitsma M, Masek M, Phan T, Tsukamoto A, Gage F, Weissman I. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Huang NN, Heppel LA. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J Cell Physiol. 1992;153:221–33. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–14. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–93. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Weissman T, Riquelme P, Ivic L, Flint A, Kriegstein A. Calcium waves propagate through radial glial cells and modulate proliferaytion in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


