Introduction
Patients with mood disorders are known to have neurocognitive deficits in many, if not most, cognitive domains.[1] In a recent paper, we showed that depressed patients on modern antidepressants had, in spite of successful treatment, residual deficits in tests of effortful attention, executive function, and information processing speed.[2] Although the cognitive impairments associated with depression are improved by effective antidepressant therapy, they do not tend to normalize.[3–6]
This general rule has not been tested, however, in comparative studies of specific antidepressants. One might expect, for example, that that antidepressants with potent noradrenergic effects would be cognitive enhancers.[7–10] In contrast, antidepressants whose primary action is serotonergic would be expected to be cognitively neutral, or less likely to “normalize” cognition in depressed patients.[11–17]
We took the opportunity to examine this proposition in a naturalistic study of depressed patients who had been treated with 7 different modern antidepressants. We wondered whether, in a real-world situation, one could demonstrate cognitive differences among patients taking different drugs. To address the question, we took advantage of a computerized neurocognitive screening battery that could administer 7 neuropsychological tests in a short period of time and generate results with millisecond accuracy. Because the test is easy to administer, it could be given, in our clinics, to virtually every patient on antidepressants.
Methods & Materials
Subjects
The subjects of this investigation were outpatients at the NC Neuropsychiatry Clinics in Chapel Hill and Charlotte, North Carolina, age 18–65 years, with the diagnosis of major depressive disorder, unipolar, nonpsychotic. The subjects were all in good health, with no concomitant neurocognitive disorders (eg, attention-deficit disorder, learning disability, chronic pain, mild cognitive impairment) and no comorbid psychiatric diagnoses. They were on no concomitant medications. Subjects were selected for inclusion in this investigation if antidepressant treatment led to optimal clinical response, in the opinion of the treating clinician, and if the patient had been discharged to routine, quarterly follow-up. All patients had to have been on a stable dose of drug for at least 4 weeks.
A search of a database of patients who had been tested with the CNS Vital Signs battery at the North Carolina Neuropsychiatry Clinics yielded no fewer than 541 patients who had been tested while on antidepressants. Eliminating patients who did not meet the above criteria, and matching patients as closely as possible for 3 equal groups on the basis of medication, age, race, and gender yielded a final sample of 81 patients (bupropion, 27; venlafaxine, 27; selective serotonin reuptake inhibitors [SSRIs], 27).
Twenty-seven controls were randomly selected from the CNS Vital Signs normal database. This database comprises individuals who are in good health with no current or past psychiatric, neurologic, or cognitive disorder, and on no current centrally active medications. The controls were matched as closely as possible for age, race, and gender. (All of the subjects, both patients and controls, were white.)
Cognitive Evaluation
The patients' neurocognitive performance was measured on a computerized battery of tests, CNS Vital Signs (CNSVS). CNSVS is a PC-based neurocognitive screening battery that comprises 7 familiar neuropsychological tests: verbal memory (VBM); visual memory (VIM); finger tapping (FTT); symbol-digit coding (SDC); the Stroop test (ST); the shifting attention test (SAT); and the continuous performance test (CPT). The test battery is self-administered in the clinic on an ordinary PC and takes about 30 minutes to complete. CNS Vital Signs generates 5 cognitive domain scores and 1 summary score (the “neurocognition index,” NCI). Group differences were assayed by MANOVA, taking as covariates patient age and gender. If significant differences between groups were detected, pairwise t tests were done to measure differences between the 3 antidepressant groups and normals.
Results
In Table 1, columns 2–5 are the domain scores NCI scores for the 3 antidepressant groups and normal controls. F scores from MANOVA and significance levels are shown in columns 6 and 7. Significant group differences exist for 4 of 5 domain scores and for the NCI. The sources of these differences were established by pairwise t tests and are presented in Table 2. The SSRI group scored significantly below normals in tests of psychomotor speed, cognitive flexibility, and reaction time. The venlafaxine group scored more poorly than normals in reaction time, a measure of information processing speed derived from the Stroop test. The bupropion group did not differ from normals in any of the cognitive domains.
Table 1.
Mean Test Scores and Difference From Normal
| Medication | SSRI | VEN | BP | NML | F | P < |
|---|---|---|---|---|---|---|
| N | 27 | 27 | 27 | 27 | ||
| Age | 43.81 | 46.11 | 44.00 | 43.85 | ||
| Male | 11 | 11 | 7 | 10 | ||
| Female | 16 | 16 | 20 | 17 | ||
| Neurocognition index | 89.77 | 94.80 | 100.00 | 98.34 | 2.59 | .023 |
| Composite memory | 96.56 | 95.50 | 100.81 | 97.70 | 2.18 | .051 |
| Psychomotor speed | 145.23 | 161.45 | 166.92 | 171.76 | 4.91 | .000 |
| Reaction time* | 697.44 | 700.02 | 649.80 | 639.17 | 4.24 | .001 |
| Cognitive flexibility | 32.04 | 38.89 | 45.07 | 43.27 | 4.24 | .001 |
| Complex attention* | 11.42 | 8.92 | 7.48 | 8.38 | 3.26 | .006 |
NOTE, Table 1. An asterisk indicates that lower scores are better.
A lower score is better.
SSRI = selective serotonin reuptake inhibitor; VEN = venlafaxine; BP = bupropion; NML = normal; F = F score
Table 2.
Pairwise t Tests Comparing Antidepressant Groups With Normals
| Paired t Test | NML vs SSRI | NML vs VEN | NML vs BP | |||
|---|---|---|---|---|---|---|
| t | P < | t | P < | t | P < | |
| Neurocognition index | 2.94 | .0049 | 1.26 | .2139 | −1.05 | .2968 |
| Composite memory | 0.49 | .6229 | 1.02 | .3144 | −1.81 | .0768 |
| Psychomotor speed | 3.93 | .0003 | 1.47 | .1483 | 1.01 | .3195 |
| Reaction time | −1.94 | .0580 | −2.03 | .0474 | −0.46 | .6446 |
| Cognitive flexibility | 3.29 | .0018 | 1.06 | .2922 | −0.85 | .4009 |
| Complex attention | −1.76 | .0846 | −0.34 | .7371 | 0.73 | .4659 |
NML = normal; SSRI = selective serotonin reuptake inhibitor; VEN = venlafaxine; BP = bupropion
Discussion
Patients with depression are subject to neuropsychological deficits in attention, memory, psychomotor speed, processing speed, and executive function. When they are treated, they perform better, but they do not perform as well as normal controls.[1,17] They improve, at least to a degree, but do not “normalize.” The data reported here suggest that how well they perform on neurocognitive testing may be a function of the antidepressant with which they are treated. What our data show is that depressed patients on bupropion perform as well as normals do on a battery of neurocognitive tests. Patients on venlafaxine and SSRIs do not.
These results are consistent with the hypothesis that cognitive benefit may occur relative to an antidepressant's norepinephrine activity, while lack of benefit may relate to its serotonergic activity. The noradrenergic/dopaminergic antidepressant bupropion is associated with normal function. The mixed serotonin/norepinephrine reuptake venlafaxine performs less well than bupropion but better than the SSRIs (Figure). This is consistent with the principle that enhanced norepinephrine metabolism is associated with better cognitive performance of a variety of neurocognitive tasks.
Figure.
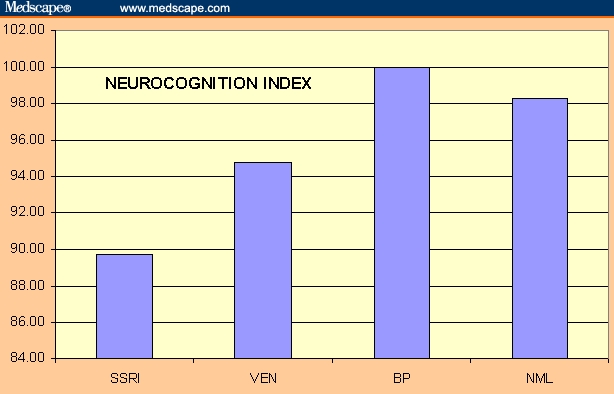
Relative summary performance of antidepressants.
A naturalistic, cross-sectional study is not an optimal environment for dealing with the complexities of the questions at hand. One would prefer to have data from a more controlled environment. The ideal way to examine drug-related changes in neurocognition is to test patients at baseline, and then to test them again after they have achieved a therapeutic result. It would also be useful to have a comparison group of patients who were treated with placebo. The severity of patients' depression at baseline and how well they have responded to treatment should have been calibrated with more precision than we have done. In addition, the only unbiased way to compare one treatment with another is to use random assignment.
Patients were not randomly assigned to medication. Antidepressants were selected on the basis of clinicians' professional good judgment. This is a flaw, but not a fatal flaw. The choice of which antidepressant to use was made by 9 experienced clinicians operating independently in 2 different clinics. No specific pattern was detected in the clinician's choice. If there was bias in drug selection, it would have worked against bupropion and venlafaxine. Psychiatrists, aware of the beneficial neurocognitive effects of bupropion, sometimes prescribe the drug for depressed patients who complain of problems with concentration or memory. Psychiatrists often prescribe venlafaxine for patients with more severe depression or who have failed to respond to SSRIs. If either of these factors had been operative in this study, one would have expected patients on bupropion or venlafaxine to do less well than patients on SSRIs.
The reason why it is important to report these data, in spite of the obvious shortcomings, is that the relative neurocognitive effects of antidepressants are rarely addressed in the medical literature, and when they are, the research has been done in laboratory settings. Not only is it important to address the issue of neurocognition with respect to the antidepressants, it is important to do so using practical tools in a real-world environment. Computerized testing allows clinicians to evaluate the cognitive effects of drug treatment in the clinic setting.
Acknowledgements & Disclosures
Drs. Gualtieri and Johnson are two of the developers of the CNS Vital Signs screening battery. Dr. Gualtieri has conducted clinical trials on behalf of AstraZeneca, Bristol-Myers Squibb, Celltech, Cephalon, Eli Lilly, GlaxoSmithKline, Medeva, Organon, Shire, Wyeth-Ayerst, and UCB Pharma. He has been a speaker for and/or consultant to Eli Lilly, GlaxoSmithKline, Pfizer, Shire, and Wyeth. This research was supported by North Carolina Neuropsychiatry, PA, in Chapel Hill and Charlotte, and by CNS Vital Signs LLC. This study was supported by a grant to the investigators from GlaxoSmithKline.
Footnotes
Readers are encouraged to respond to the author at tg@ncneuropsych.com or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
Contributor Information
C. Thomas Gualtieri, North Carolina Neuropsychiatry Clinics, Chapel Hill, North Carolina; Scientific Director, CNS Vital Signs, LLP, Chapel Hill, North Carolina Author's Email: tg@ncneuropsych.com.
Lynda G. Johnson, North Carolina Neuropsychiatry Clinics, Chapel Hill, North Carolina.
References
- 1.Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. J Neuropsychiatry Clin Neurosci. 2006;18:217–225. doi: 10.1176/jnp.2006.18.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry. 1996;153:490–496. doi: 10.1176/ajp.153.4.490. [DOI] [PubMed] [Google Scholar]
- 3.Doraiswamy PM, Krishnan KR, Oxman T, et al. Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol A Biol Sci Med Sci. 2003;58:M1137–M1144. doi: 10.1093/gerona/58.12.m1137. [DOI] [PubMed] [Google Scholar]
- 4.Nebes RD, Pollock BG, Houck P, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 5.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 6.Frasch K, Bretschneider S, Bullacher C, Hess R, Wittek R, Neumann NU. [Do cognitive deficits in depressive disorders remit?] Psychiatr Prax. 2000;27:291–295. [PubMed] [Google Scholar]
- 7.Clay TH, Gualtieri CT, Evans RW, Gullion CM. Clinical and neuropsychological effects of the novel antidepressant bupropion. Psychopharmacol Bull. 1988;24:143–148. [PubMed] [Google Scholar]
- 8.Hindmarch I, Kerr J. Behavioural toxicity of antidepressants with particular reference to moclobemide. Psychopharmacology (Berl) 1992;106:49–55. doi: 10.1007/BF02246236. [DOI] [PubMed] [Google Scholar]
- 9.Kerr JS, Powell J, Hindmarch I. The effects of reboxetine and amitriptyline, with and without alcohol on cognitive function and psychomotor performance. Br J Clin Pharmacol. 1996;42:239–241. doi: 10.1046/j.1365-2125.1996.39016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furlan PM, Kallan MJ, Ten Have T, Pollock BG, Katz I, Lucki I. Cognitive and psychomotor effects of paroxetine and sertraline on healthy elderly volunteers. Am J Geriatr Psychiatr. 2001;9:429–438. [PubMed] [Google Scholar]
- 11.Cassano GB, Puca F, Scapicchio PL, Trabucchi M. Paroxetine and fluoxetine effects on mood and cognitive functions in depressed nondemented elderly patients. J Clin Psychiatry. 2002;63:396–402. [PubMed] [Google Scholar]
- 12.Schmitt JA, Kruizinga MJ, Riedel WJ. Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. J Psychopharmacol. 2001;15:173–179. doi: 10.1177/026988110101500304. [DOI] [PubMed] [Google Scholar]
- 13.Hindmarch I, Harrison C, Shillingford CA. An investigation of the effects of lofepramine, nomifensine, amitriptyline and placebo on aspects of memory and psychomotor performance related to car driving. Int Clin Psychopharmacol. 1988;3:157–165. doi: 10.1097/00004850-198804000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Hindmarch I, Bhatti JZ. Psychopharmacological effects of sertraline in normal, healthy volunteers. Eur J Clin Pharmacol. 1988;35:221–223. doi: 10.1007/BF00609258. [DOI] [PubMed] [Google Scholar]
- 15.Lader M, Melhuish A, Frcka G, Fredricson Overo K, Christensen V. The effects of citalopram in single and repeated doses and with alcohol on physiological and psychological measures in healthy subjects. Eur J Clin Pharmacol. 1986;31:183–190. doi: 10.1007/BF00606656. [DOI] [PubMed] [Google Scholar]
- 16.Bangs ME, Petti TA, Janus MD. Fluoxetine-induced memory impairment in an adolescent. J Am Acad Child Adolesc Psychiatry. 1994;33:1303–1306. doi: 10.1097/00004583-199411000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Gualtieri CT, Johnson LG, Benedict KB. Drug sensitivity of a computerized neurocognitive test battery. Program and abstracts of the 32nd Annual Meeting of the International Neuropsychological Society; February 4–7, 2004; Baltimore, Maryland. Poster Session 6: Measures of Broad Neuropsychological Functions. [Google Scholar]


