Abstract
The activity of a novel enaminone derivative of 4–hydroxyquinoline, BDHQ, was screened for its effectiveness against murine schistosomiasis by electron microscopy and parasitologic studies. The correlation of these studies with serum levels of IFN–gamma and IgE is described. Two groups of 10 mice each were treated with different doses of BDHQ, and their results were correlated with the control and praziquantel (PZQ)–treated groups. Parasitologic study revealed significant reduction in mature worms and tissue egg loads in BDHQ– and PZQ–treated groups, whereas immature worms revealed significant reduction in BDHQ groups only. The group treated with a higher dose of BDHQ showed significant reductions in intestinal ova count when compared with the PZQ–treated group. Ultrastructural examination of the worm revealed significant degeneration of the spines and tegument in all treated groups, while the genital system was affected in BDHQ–treated groups only. BDHQ showed considerable effect on cellular activation where serum levels of IFN–gamma were significantly increased in comparison to control, while anti–soluble worm antigen preparation (SWAP) IgE was significantly increased in comparison to both the control and PZQ–treated groups. Ultrastructural examination revealed cellular activation in buffy coat and the liver in both the BDHQ– and PZQ–treated groups in comparison to the untreated one, whereas in the bone marrow and spleen, evidence of cellular activation was remarkable in the BDHQ–treated groups. In conclusion, BDHQ exhibits high levels of activity against adult and juvenile stages of these parasites, which may be due to its mixed cellular and humoral immunologic mechanisms, as demonstrated by the significant increase of serum levels of IgE and IFN–gamma shown on electron microscopy. Therefore, our results support the comparative advantage that BDHQ has over PZQ.
Introduction
It is estimated that about 200 million people worldwide are currently affected by schistosomiasis, a disease caused by flatworms belonging to the genus Schistosoma. The disease is usually chronic and debilitating, with severe consequences to the urinary tract when S hematobium is the organism involved and major damage to the liver and intestine when S mansoni or S japoniciun is involved. Only 3 drugs are in current use (metrifonate, oxamniquine, and praziquantel [PZQ]), and PZQ may be the only one destined to remain on the market. To be left with only 1 drug easily available against schistosomiasis is clearly a very dangerous situation, especially in view of the very recent report on PZQ–resistant schistosomes. This would not be surprising in countries like Egypt, where the drug has been used aggressively for more than 10 years. In addition, PZQ does not prevent reinfection and sometimes even enhances the rate of reinfection, especially in young adults.[1] Moreover, PZQ is not ovicidal, nor is it effective against young stages of the parasite.[2] Because of phenomena such as the development of resistance, it remains an urgent necessity to find new compounds that show particularly good efficacy against schistosomiasis.
Th cell subsets originate from CD4+ T cell precursors and are defined by the cytokines they produce.[3] These subsets have been shown to play a central role in the control and regulation of many immune responses, including those directed against parasitic infections such as Leishmania major and Schistosoma mansoni. In murine models of S mansoni infection, a well–defined Th subset dichotomy has been reported in which T helper 1 (Th1) produces interferon gamma (IFN–gamma) and tumor necrosis factor–alpha (TNF–alpha), which activate macrophages as part of a delayed type hypersensitivity reaction. The reaction was reported to provide protection against S mansoni infection in mice.[4] Th2 lymphocytes are known to produce IL–4, IL–5, IL–10, and IL–13, which are essential for the production by B cells of IgE, IgG1, IgA, and IgM antibody isotypes and eosinophil and mast cell activation. Th2–type cells are believed to be essential in human protection against schistosome infections but have been shown to play a role in pathology, which accompanies the deposition of eggs in the tissues of S mansoni–infected mice.[5] A series of immunoepidemiologic studies involving the follow–up of drug–cured S mansoni and S haematobium patients who subsequently became reinfected has shown that a proportion of these subjects acquire partial immunity to reinfection, and that this immunity appears to be dependent upon specific IgE antibodies.[6]
Recently, a series of novel synthesized enaminones, derived from 3–(un)substituted 4–hydroxyquinolin–2(1H)–ones, were assayed for their molluscicidal activities against Biomphalaria alexandrina and Lymnaea natalensis snails. From this series, 1–butyl–3–[2 ethyl–3–(dimethylamino) propyl–2–enoyl]–4–hydroxyquinolin–2(1H)–one (BDHQ) showed more potency against hatchability of B alexandrina egg masses, the infection rate, and the prepatent period of the snails. In addition, this derivative revealed potential larvicidal effects (100% mortality) on both miracidia and cercariae of S mansoni with reduced exposure time. This active derivative was also examined against Daphnia magna, and their nontoxic effect at LD/50 suggests that this compound is environmentally safe and can play important roles as molluscicides and larvicides.[7] BDHQ can thus be considered to exhibit unique antischistosomal activity.
In the present study, we used electron microscopy and parasitologic study to screen the novel enaminone derivative of 4–hydroxyquinolinone, BDHQ, for its potential activity against murine schistosomiasis and correlate this study with serum levels of IFN–gamma and IgE.
Materials and Methods
Schistosome Infections
S mansoni cercariae were provided by the Malacology Lab of the Schistosomiasis Unit (SBSP), Theodor Bilharz Research Institute (TBRI), where laboratory–bred B alexandrina are maintained. Infection was performed by tail immersion using 100 S mansoni cercariae to each mouse. Laboratory–bred male albino mice of the CDI strain, each weighing 18–20 grams, were used in this study. Experimental animals were kept in an air–conditioned room at 21°C and received food containing 24% protein.
Pharmacologic Agents
Praziquantel. PZQ (EMBAY 8440) was purchased from Bayer (Leverkusen, Germany) and E. Merck (Darmstadt, Germany).
Synthesis of BDHQ
As outlined in Figure 1, condensation of N–butylaniline with excess diethyl malonate led to the formation of 6–butyl–4–hydroxypyranoquinolinedione BHPQ. Alkaline hydrolysis of the product BHPQ, using 15% sodium hydroxide, furnished the corresponding 3–acetyl–1–butyl–4–hydroxyquinolin–2(1H)–one (ABHQ).[8] Thermal condensation of ABHQ with dimethylformamide–dimethylacetal (DMF–DMA) afforded1–butyl–3–[2E–3–(dimethylamino)prop–2–enoyl]–4–hydroxy–quinoline–2(1H)–one (BDHQ), in 81% yield.[7] IR (frequency), mass, and elemental analyses supported the structure of BDHQ. Besides, 1H NMR revealed that BDHQ assumes an E–form where the coupling constant of the olefinic protons is 13.5 Hz.
Figure 1.
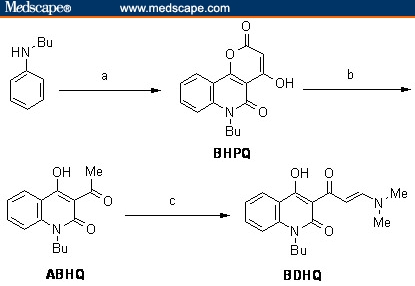
Reagents and conditions: (a) diethyl malonate, 220°C, 4 hrs; (b) aq. NaOH (15%), reflux, 2 hrs; (c) DMF–DMA, toluene, 110°C, 2 hrs.
Experimental Design
Chemistry
Melting point is uncorrected and was determined in an open capillary tube on a digital Gallen–kamp MFB–595 apparatus. Infrared spectrum was recorded on a Perkin–Elmer 1650 FT–IR spectrophotometer, using a sample in KBr pellet. 1H NMR spectrum was recorded on Varian Gemini (200 MHz), using TMS as an internal standard and CDCl3 as solvents. Mass spectrum was taken on a Shimadzu GCMS QP–1000EX instrument by direct inlet technique at beam energy 70 eV. Elemental microanalysis was performed on a Perkin–Elmer 2400 analyzer.
1–Butyl–3–[2E–3–(dimethylamino)prop–2–enoyl]–4–hydroxyquinolin–2(1H)–one (BDHQ)
DMF–DMA (1.4 mL, 10 mmol) was added to a solution of acetylquinolinone ABHQ, in dry toluene (50 mL), and the reaction mixture was heated under reflux for 2 hours. The excess solvent was evaporated in vacuum and the residual material was triturated with petroleum ether (60–80°C) (25 mL), filtered off and crystallized from benzene. Yield 2.54 g (81 %); mp 126–7°C. IR (KBr): νmax 3131, 3080, 2956, 2924, 2860, 1642 (C = O), 1622, 1526, 1498 cm–1; 1H NMR (200 MHz, CDCl3): δ0.96 (t, 3H, N–(CH2)3CH3), 1.31 (m, 4H, N–CH2(CH2)2CH3), 3.02 (s, 3H, N(CH3)2), 3.21 (s, 3H, N(CH3)2), 3.87 (t, 2H, N–CH2(CH2)2CH3), 7.08 (d, J = 13.5 Hz, 1H, COCH=CH–N), 7.21–7.32 (m, 2H, Harom), 7.59 (t, 1H, 7–Hquinolinone), 8.09 (d, J = 13.5 Hz, 1H, COCH=CH–N), 8.22 (d, J = 8 Hz, 1H, 5–Hquinolinone); MS: m/z (I %) 314 (M+, 28), 270 (100). Anal. calculated for C18H22N2O3 (314.39): C, 68.77; H, 7.05; N, 8.91. Results: C, 68.68; H, 6.94; N, 8.56.
Biology
The mice were divided into 4 groups, 10 animals each. Group I (infected untreated controls): 5 mice were sacrificed after 3 weeks (so that the schistosomules could be counted) and the other 5 after 6 weeks (so the adult worms could be counted). Group II: 5 mice were treated at the third week post infection through oral use of an esophogeal tube with BDHQ (10 mg/mL) for 2 consecutive days (0.5 mL × 2), and the other 5 mice were treated at the sixth week post infection as above. Group III: Same as group II, but we used a higher dose of BDHQ (1 mL × 2). Group IV: 5 mice were treated at the third week post infection with PZQ for 2 consecutive days (2 × 500 mg/kg), and the other 5 mice were treated at the sixth week post infection as above. All groups were sacrificed 2 weeks after treatment.
Sample Collection and Preparation
After the mice were scarified by cervical dislocation, some of the blood was collected in tubes containing EDTA as anticoagulant and centrifuged to obtain the buffy coat, and some of the blood was collected in tubes without anticoagulant and centrifuged to obtain the serum, which was frozen at –20°C until tested. The hepatic and portomesenteric vessels were perfused to study worm load and sex. Worms, sections of the livers and spleens, buffy coat, and bone marrow from the femur bones were fixed in 4% glutaraldehyde with sodium cacodylate for electron microscopic examination. The rest of the liver and intestinal fragment tissues were used for the recovery of eggs and determination of the percentage of eggs in each developmental stage.
Worm Burden and Distribution
Worm burden and sex were studied after perfusion of the hepatoportomesenteric vessels.[9]
Tissue Egg Load
The number of eggs per gram of tissue was studied by weighing 0.5 g of liver or small intestine, which was then digested and incubated overnight in 5% KOH. The hepatic and intestinal tissue egg loads were determined by multiplying the average number of eggs in each 1–mL sample by the total volume of KOH and then dividing that value by the weight of the sample to yield the number of eggs per gram of tissue.[10]
Electron Microscopic Examination
The collected samples were transferred after 2 hours from glutaraldehyde and sodium cacodylate to be fixed in 2% osmium tetraoxide, dehydrated with increasing concentrations of alcohol, and embedded in epoxy resin according to the technique of Grimaud and colleagues.[11]
Semi–thin and ultra–thin section sections were cut with a Leika ultramicrotome. Ultra–thin sections were contrasted with uranyl acetate and lead citrate stains and were then examined by Phillips EM–208 electron microscope.
IFN–gamma Assays by ELISA
The collected sera were tested with commercially available enzyme linked immunosorbent assay (ELISA) kits (Quantikine M mouse IFN–gamma, Diaclone Research USR, United Kingdom). The procedure was done according to the manufacturer's instruction. Standard curves were generated from known concentrations of cytokine provided by the manufacturer. Standard curves were then used to predict the quantity of cytokine based on the level of spectrophotometric absorbance for each sample using regression analysis.
Measurement of Serum Anti–SWAP IgE
Measurement of serum level of IgE by ELISA was based on the original method of Engvall and Perlman.[12] For assessment of serum Ig E, Immulon 4 (Dynatech Laboratories, Chantilly, Virginia, USA) microtiter plates were coated overnight at 4°C with SWAP (1 mcg in 50 mcl/well) diluted in PBS. Plates were blocked with 200 mcl 5% nonfat dry milk/PBS for 2 hours at 37°C. The blocking solution was aspirated and the wells washed 6 times with PBS/0.05% Tween–20 (Sigma) (St. Louis, Missouri, USA). Individual mouse serum was diluted 1/100 in 1% BSA/PBS, and 50 mcl was added to appropriate wells. Plates were incubated at 37°C for 90 minutes and then washed 6 times with PBS/0.05% Tween–20. Fifty microliters of isotype–specific horseradish peroxidase–conjugated rabbit anti–mouse Abs in 1% BSA/PBS diluted at 1/500 were added to the wells and incubated at 37°C for 2 hours. Wells were again washed 6 times with PBS/0.05% Tween–20 and 100 mcl of (2,2'–azino–di(3–ethylbenzthiazolinesulfonate) (ABTS: H2O2 substrate [Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA]) was added and the reactions developed in the dark at room temperature for 20 to 30 minutes. Absorbance at 405 nm was determined using an ELISA reader (Bio–Rad Microplate Reader, Richmond, California, USA).
Statistical Analysis
The data are presented as mean ± standard error of mean (X ± SE). The means of the different groups were compared globally using the analysis of variance (ANOVA). The data were considered significant if P values were ≤ .05.
Results
All the mice survived in good health without weight loss until the end of the experiment.
Parasitologic Study (Table 1 and Table 2)
Table 1.
Worm Burden in S mansoni—Infected Mice Treated With BDHQ and PZQ vs Infected Untreated Control Group
| Animal Group | Drug Dose | Mean Worm Burden ± SD | Immature |
|---|---|---|---|
| Group I (n = 10) | _ | 31.4 ± 0.85 | 36 ± 1.41 |
| Group II (n = 10) | 2 × 0.5 mL | 8.7 ± 1.11* | 20 ± 4.10† |
| Group III (n = 10 | 2 × 1 mL | 4.1 ± 0.67* | 20 ± 4.10† |
| Group IV (n = 10) | 2 × 500 mg/kg | 1.22 ± 0.72* | 34.5 ± 4.5 |
Highly significant difference from group 1 (P < .001)
Significant reduction from groups 1 and IV (P < .05)
Table 2.
Ova Count in S mansoni—Infected Mice Treated With BDHQ and PZQ vs Infected Untreated Control Group
| Animal Group | Mean Ova Count/Gram Tissue | |
|---|---|---|
| Liver ± SD | Intestine ± SD | |
| Group I (n = 10) | 18667 ± 144 | 29654 ± 935 |
| Group II (n = 10) | 8380 ± 109.5* | 7110 ± 841.49* |
| Group III (n = 10) | 5120 ± 4.4* | 1040 ± 140.79*† |
| Group IV (n = 10) | 4570.37 ± 172.27* | 3524.41 ± 2195.81* |
Highly significant difference from group 1 (P < .001)
Significant reduction from groups 1 and IV (P < .05)
PZQ–and BDHQ–treated groups caused highly significant reductions (P < .001) in mature worm loads and tissue egg loads compared with control, while BDHQ–treated groups only showed significant reductions in immature worm compared with control and PZQ–treated groups (P < .05). The group treated with a higher dose showed significant reductions in intestinal ova count compared with the PZQ–treated group (P < .05).
IFN–gamma and Anti–SWAP IgE (Table 3, Figures 1 and 2)
Table 3.
Serum Levels of IFN—gamma and Anti—SWAP IgE in S mansoni—Infected Mice Treated With BDHQ and PZQ vs Infected Untreated Control Group
| Animal Group | IFN—gamma (pg/mL) | Anti—Swap IgE (mcg/mL) |
|---|---|---|
| Group I (n=10) | 100 ± 2.45 | 0.25 ± 0.06 |
| Group II (n=10) | 118 ± 6.87* | 0.39 ± 0.031*† |
| Group III (n=10) | 125 ± 5.33* | 0.41 ± 0.1*† |
| Group IV (n=10) | 120 ± 3.33* | 0.32 ± 0.05* |
Significant difference from group I (P < .05)
Significant difference from group IV (P < .05).
Figure 2.
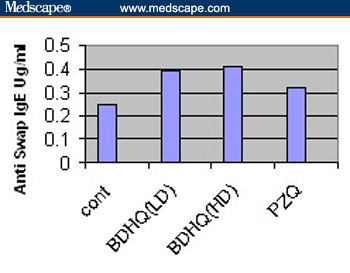
Serum levels of anti–SWAP IgE in Schistosoma mansoni–infected mice treated with BDHQ at 2 different doses and praziquantel compared with infected untreated controls.
On comparison of BDHQ with other groups, we found a significant increase in serum levels of IFN–gamma compared with controls (P < .05), and the difference between the BDHQ– and PZQ–treated groups was nonsignificant. With regard to anti–SWAP IgE, a significant increase was observed compared with controls and PZQ–treated groups (P < .05).
Ultrastructural Study
Worms. Ultrastructural examination of the worms revealed detachment and implantation of the spines within vesiculated and degenerated tegument in all treated groups in comparison to untreated groups (Figures 3–6), while the genital system was affected in the BDHQ–treated group only in the form of pycnosis of germinal vesicles of the oocytes and necrosis of the follicular epithelium in female worms (Figure 7) and necrosis of spermatocyte in male worms (Figure 8).
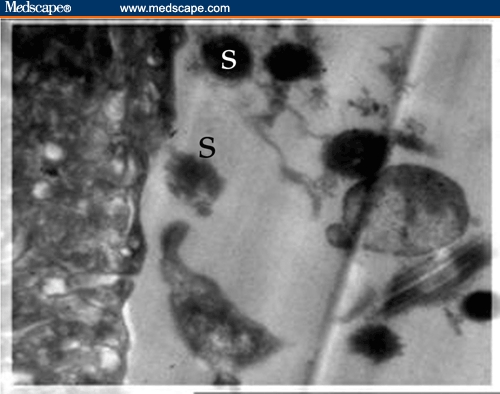
Figure 3.

Electron micrograph of the tegument of untreated Schistosoma mansoni showing the pointed spines (S) (X 2000).
Figure 6.
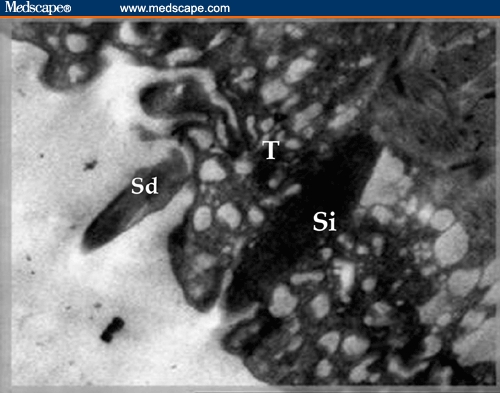
Electron micrograph of praziquantel–treated Schistosoma mansoni worm showing detachment of one spine (Sd) and implantation of the other (Si) within severely vesiculated tegument (T) (X 3000).
Figure 7.
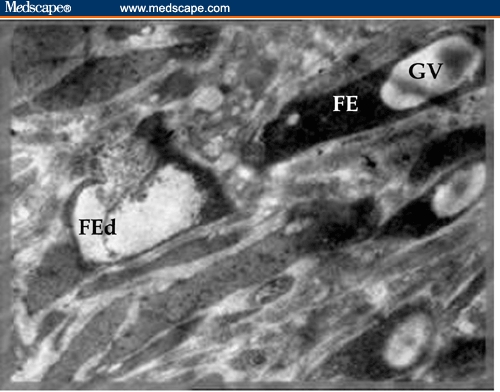
Electron micrograph of BDHQ–treated female Schistosoma mansoni worm showing pycnosis of germinal vesicles of all the oocytes and necrosis of follicular epithelium of the left one (FEd) (X 3000).
Figure 8.

Electron micrograph of BDHQ–treated male Schistosoma mansoni worm showing vaculation and necrosis of spermatocyte (S) (X 5000).
Buffy Coat, Bone Marrow, Liver, and Spleen. Ultrastructural examination showed evidence of cellular activation in the buffy coat and liver in both BDHQ– and PZQ–treated groups in comparison to untreated groups, while in the bone marrow and spleen, the evidence of cellular activation was remarkable in BDHQ–treated groups. In buffy coat examination of all treated groups, monocytes revealed evidence of activation represented by a large number of extending pseudopodia (Figure 9) and the activation of lymphocytes represented by thinly distributed chromatin in most parts of the nucleus, vacuolization of the cytoplasm, and a large number of mitochondria (Figure 10). Bone marrow examination showed evidence of promonocyte activation in BDHQ–treated groups (Figure 11), while eosinophil activation could be detected in all treated groups (Figures 12 and 13). Evidence of lymphocyte activation was observed in both PZQ–and BDHQ–treated livers (Figure 14 and 15). Spleen examination in BDHQ–treated groups showed increases in follicular dendritic cells –– with irregular nucleus that was poor in heterochromatin and thin sheets of cytoplasmic extensions –– and their intimate contact with the lymphocytes could be observed (Figure 16). Remarkable increases in longitudinal intracellular contractile filaments in the endothelial cells lining the splenic sinus and its fenestration could be observed (Figure 17).
Figure 9.
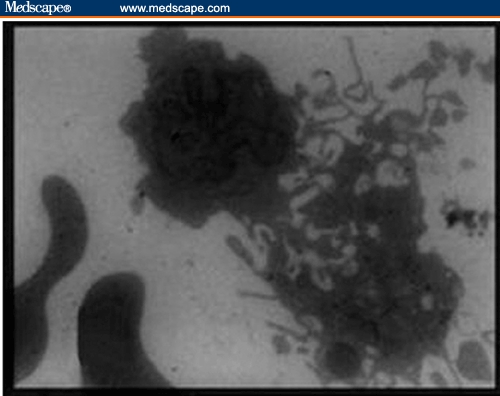
Electron micrograph of 2 monocytes in the peripheral blood of BDHQ–treated mouse revealed evidence of activation represented by large number of extending pseudopodia (x 2500).
Figure 10.
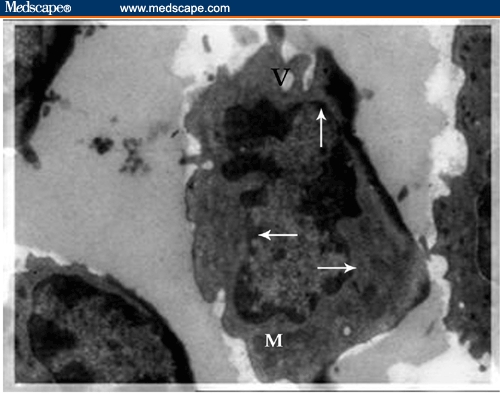
Electron micrograph of activated lymphocyte in the peripheral blood of praziquantel–treated mouse. The cell is characterized by thinly distributed chromatin in most parts of the nucleus (arrow), vacuolization of the cytoplasm (V), and a large number of mitochondria (M) (x 2500.)
Figure 11.
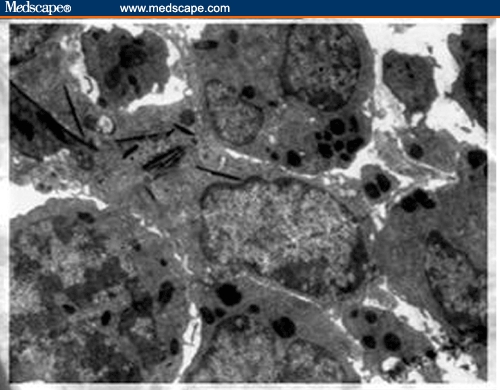
Electron micrograph of a promonocyte in bone marrow of BDHQ–treated mouse. The nucleus has fine chromatin and is situated at one end of the cell. Bundles of filaments are prominent in the cytoplasm, which are presumably related to its property of motility and active endocytosis (X 3600).
Figure 12.
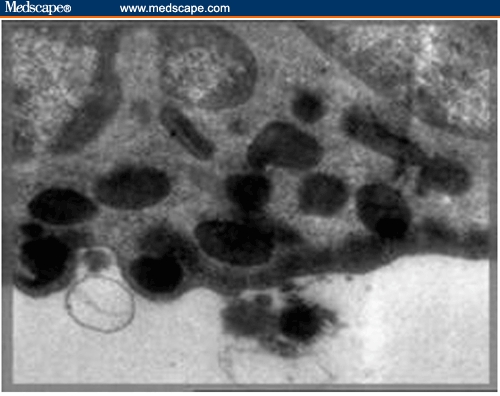
High magnification showing the process of discharge of the eosinophilic granules in the bone marrow of a mouse treated with praziquantel (x 8000).
Figure 13.
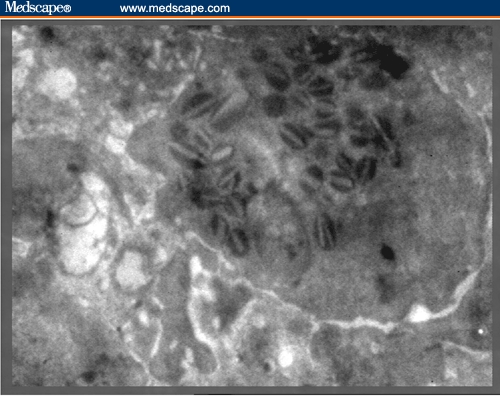
Electron micrograph of degranulated eosinophil in the bone marrow of BDHQ–treated mouse (x 4500).
Figure 14.
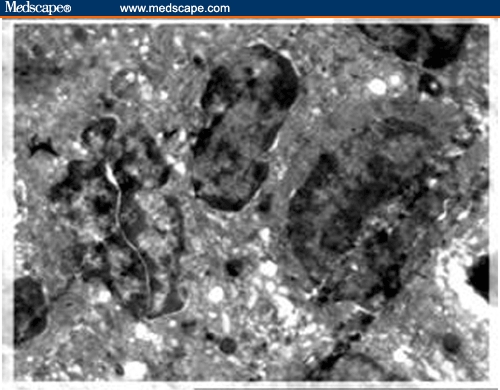
Ultrastructure of 3 activated lymphocytes characterized by thinly distributed chromatin in the liver of Schistosoma mansoni–infected mouse treated with praziquantel; they are in intimate contact and the left one is in mitosis (X 4000).
Figure 15.
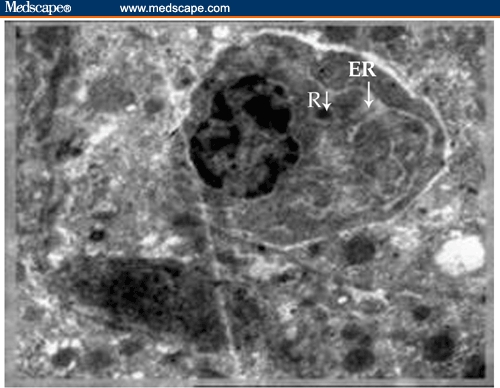
Ultrastructure of activated lymphocyte (plasmablast) in intimate contact with the fibroblast in the liver of Schistosoma mansoni–infected mouse treated with BDHQ. The plasmablast shows a round and small eccentric nucleus in relation to the cell size. The cytoplasm is filled with dilated endoplasmic reticulum (ER) and a few remaining free ribosomes (R). (X 5000)
Figure 16.
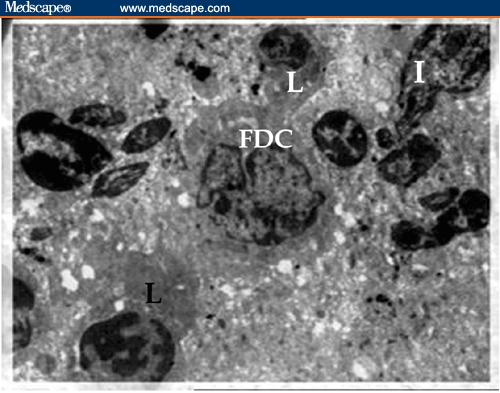
Electron micrographs of spleen of BDHQ–treated mouse showing the tightly packed lymphocytes in the central area of the periarteriolar lymphatic sheath (PALS); they are in intimate contact with the interdigitating cells (I). Junction between follicular dendritic cell (FDC), with irregular nucleus that is poor in heterochromatin and thin–sheet cytoplasmic extensions, and 2 lymphocytes (L) were detected (X 1800).
Figure 17.
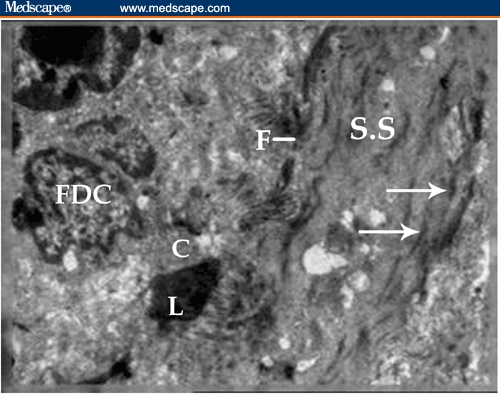
Longitudinal section of the splenic sinus (S.S.) in BDHQ–treated mouse showing the increase of longitudinal intracellular contractile filaments (arrow) in the endothelial cells lining the splenic sinus; fenestration of the sinus (F) could be observed. Follicular dendritic cells (FDC) are sending thin–sheet cytoplasmic extensions (c) to the adjacent activated lymphocyte (L) that is going to enter into the sinus (X 1800).
Discussion
For schistosomiasis, vaccine is nonexistent and drugs remain the mainstay of disease control. However, the current drug repertoire is limited and/or inadequate, and the problem is being further exacerbated by the emergence of drug resistance. Because of this phenomenon, it remains an urgent necessity to find new compounds that show particularly good efficacy against schistosomiasis with minimal toxicity.
1–Butyl–3–[2E–3–(dimethylamino)prop–2–enoyl]–4–hydroxyquinoline–2(1H)–one (BDHQ) showed more potency against hatchability of B alexandrina egg masses, the infection rate, and prepatent period of the snails. In addition, this derivative revealed potential larvicidal effects (100% mortality) on both miracidia and cercariae of Schistosoma mansoni at reduced exposure time.[7]
In the present study, we tried to screen –– by electron microscopy and parasitologic study –– BDHQ for its potential activity against S mansoni in a murine model of infection, and we correlated this study with serum levels of IFN–gamma and IgE to evaluate both humoral and cellular immune responses. We compared its effect with that of PZQ, which is the agent of choice in the treatment of schistosomiasis as it is highly effective against both species of schistosome and has low toxicity.[2]
In this study, the various parasitologic criteria indicated the in vivo antischistosomal effects of 2 BDHQ dosing regimens: both doses caused significant reductions in mature and immature worm loads and tissue egg loads compared with control and in immature worms compared with the PZQ–treated group, while the group treated with a higher dose showed significant reductions in intestinal ova count compared with the PZQ–treated group.
So both mature and immature worms are targets for the action of BDHQ, in contrast to the effects of the recognized antischistosomal drug PZQ, which kills only adult schistosomes.[2]
Ultrastructural study of the worms revealed a significant destructive effect of either BDHQ or PZQ on worm tegument, whereas BDHQ only has significant destructive effects on the female and male genital systems in the form of pycnosis of germinal vesicles of the oocytes and vaculation and necrosis of spermatocyte. Jürg and colleagues[13] reported that PZQ could produce contraction and paralysis of helminths by affecting membrane permeability to calcium.
Gamma interferon is a Th1 cytokine that can activate macrophages to produce NO and other inflammatory mediators.[14] Zhang and colleagues[15] found that IFN–gamma may suppress liver fibrogenesis induced by upregulation of TGF–beta1 in mice infected by Schistosoma, so it may be effective in the treatment of hepatic fibrosis. Similarly, Henri and colleagues[16] reported that IFN–gamma is certainly the most powerful and most active antifibrogenic cytokine in the experimental schistosome egg granuloma, and they also observed that IFN levels are inversely related to infection associated with periportal fibrosis, so the high rate of infections may contribute to periportal fibrosis by downmodulating IFN–gamma.
In our study, serum levels of IFN–gamma significantly increased in BDHQ–treated groups in comparison to the control group, and no significant difference was detected between the BDHQ– and PZQ–treated groups. The rise of serum levels in the treated groups could be attributed to an overall activation of the immune system, as suggested by Lins de Morais and colleagues.[17] Ribeiro de Jesus and colleagues[18] supported the role of IFN–gamma production in the protective response in human beings by showing higher levels of this cytokine in partially or completely resistant subjects, and they also suggested that IFN–gamma in humans stimulates the production of IgG2, which may be involved in the protective immunity.
With regard to serum levels of anti–SWAP IgE, a significant increase was observed in the BDHQ–treated groups compared with the control and the PZQ–treated groups. Naus and colleagues[19] reported that IgE responses against the adult worm were associated with resistance to reinfection after chemotherapy. Previous studies of S mansoni in Senegal and Kenya[20] showed no association between specific IgE responses and pretreatment infection intensity, while in studies in Brazil and the Sudan,[21] a positive correlation was found. Capron[22] found that specific IgE, interacting with eosinophils and phagocytic cells, has been demonstrated to be effective in schistosomula destruction in vitro and may be an important immune defense mechanism in the skin, where these cell types are found at the time of cercarial penetration. Bahia–Oliveira and colleagues[23] and Demeure and colleagues[6] found that high levels of both specific IgE in sera and IFN–gamma in antigen–stimulated peripheral blood mononuclear cell cultures were associated with high resistance to reinfection.
In this study, our results of IgE and IFN–gamma in sera were confirmed by the ultrastructural study of peripheral blood, bone marrow, liver, and spleen, which imply evident features of cellular activation in treated groups compared with the infected, untreated group. Also, the increasing number of follicular dendritic cells in the BDHQ–treated spleens, in addition to promonocyte activation in the bone marrow and activated monocytes in peripheral blood, might be consequence of the significant increase of IFN–gamma secreted by activated T lymphocytes by the novel hydroxyquinoline derivative (BDHQ). These findings are supported by Sher and colleagues,[4] who reported the role of T helper 1 (Th1) in production of IFN–gamma that activate macrophages as part of a delayed type hypersensitivity reaction, the reaction was reported to provide protection against S mansoni infection in mice. On the other hand, Biggelaar and colleagues[24] studied the role of dendritic cells in chronic schistosomiasis and reported that schistosome–specific T–helper cells are present in the periphery but are functionally hyporesponsive during active infection with schistosomes and cytokine responses representing the Th1 and, more importantly, Th2 types can be restored by dendritic cells. Also, the intimate contact of dendritic cells with lymphocytes, as seen on electron microscopy, was confirmed by Colley and colleagues,[25] who reported the role of dendritic cells as critical antigen–presenting cells for the induction and control of immune responses and demonstrated that schistosomes can induce elevated percentages of PD–L2 (B7–DC), which is a regulatory ligand on subpopulations of dendritic cells and binds to the coregulatory receptor PD–1, present on some activated T lymphocytes, leading to downregulation. In addition, BDHQ–treated spleens revealed a remarkable increase of longitudinal intracellular contractile filaments in the endothelial cells lining the splenic sinus, so the interendothelial slits that are normally narrow or even closed[26] are forced apart by endothelial contraction, thereby increasing the blood flow from the filtration bed and into the vein through these slits. The increased longitudinal intracellular contractile filaments in the endothelial cells may be attributable to the increased stimulation of nitric oxide synthesis in endothelial cells by hydroxyquinoline via an impairment of iron metabolism that stimulates glutathiolation of cytoskeletal and mitochondrial proteins,[27] in addition to the role of hydroxyquinoline in Mg2+ and Zn2+ regulation that allow dynamic regulation of actin microfilament organization.[28]
Conclusions
In addition to the favorable safety profile of BDHQ, its activity against schistosomes was recorded over a reasonably wide range of the life cycle since reductions in mean number of ova, immature stages and worm burden were enhanced especially in those treated with a high dose (20 mg × 2) of BDHQ. Its underlying mechanism(s) may be due to a direct assault on the tegument and the genital systems of male and female worms, as detected by electron microscopy, in addition to its role in immunologic mechanisms with mixed cellular and humoral responses, as detected by the significant increase in serum levels of IgE and IFN–gamma (as a result of remarkable cellular activation) on electron microscopy.
These results encourage us and emphasize the need to conduct additional clinical studies of this new drug in different epidemiologic settings and at higher doses. Future trials are needed to attempt to eradicate the immature form completely and to study the effect of BDHQ on Schistosoma hematobium.
Figure 4.
Electron micrograph of the tegument of BDHQ–treated Schistosoma mansoni showing complete detachment of the spines (S) (X2000).
Figure 5.

Electron micrograph of BDHQ–treated Schistosoma mansoni worm showing implantation of the spines within vesiculated tegument (T) (X 3000).
Funding Information
This work was supported by the Theodore Bilharz Research Institute.
Footnotes
Readers are encouraged to respond to the author at amira7121956@yahoo.com or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
Contributor Information
Amal M. El-Shennawy, Parasitology Department, Theodore Bilharz Research Institute, Imbaba, Giza.
Amira H. Mohamed, Electron Microscopy (Haematology) Department, Theodore Bilharz Research Institute, Imbaba, Egypt.
Mohamed Abass, Ain Shams University, Roxy, Egypt Author's Email: amira7121956@yahoo.com.
References
- 1.Butterworth AE, Dunne DW, Fulford AJ, Ouma JH, Sturrock RF. Immunity and morbidity in Schistosoma mansoni infection: quantitative aspects. Am J Trop Med Hyg. 1996;55:109–115. doi: 10.4269/ajtmh.1996.55.109. [DOI] [PubMed] [Google Scholar]
- 2.Shaw MK. Schistosoma mansoni: stage–dependent damage after in vivo treatment with praziquantel. Parasitology. 1990;100:65–72. doi: 10.1017/s0031182000060121. [DOI] [PubMed] [Google Scholar]
- 3.Mossman TR. Cytokine secretion patterns and cross–regulation of T cell subsets. regulation of immune responses by T cells with different cytokine secretion phenotypes: role of a new cytokine, cytokine synthesis inhibitory factor (IL10) Immunol Res. 1991;10:183. doi: 10.1159/000235340. [DOI] [PubMed] [Google Scholar]
- 4.Sher A, James S, Correa–Oliveira R, Hieny S, Pearce E. Schistosome vaccines: current progress and future prospects. Parasitology. 1992;98:S61–S68. doi: 10.1017/s0031182000072255. [DOI] [PubMed] [Google Scholar]
- 5.Mwinzi PN, Karanja DM, Colley DG, Orago AS, Secor WE. Cellular immune responses of schistosomiasis patients are altered by human immunodeficiency virus type 1 coinfection. J Infect Dis. 2001;184:488–496. doi: 10.1086/322783. [DOI] [PubMed] [Google Scholar]
- 6.Demeure CE, Rihet P, Abel L, Quattara M, Bourgois A, Dessein AJ. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993;168:1000–1008. doi: 10.1093/infdis/168.4.1000. [DOI] [PubMed] [Google Scholar]
- 7.Abass M, Mostafa B. Synthesis and evaluation of molluscicidal and larvicidal activities of some novel enaminones derived from 4–hydroxyquinolinones. Bioorg Med Chem. 2005;13:6133–6144. doi: 10.1016/j.bmc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Kappe T, Aigner R, Hohengassner P, Stadibauer W. Syntheses of 3–acyl–4–hydroxy–2 (IH)quinolones. J Prakt Chem. 1994;336:596–601. [Google Scholar]
- 9.Duvall RH, DeWitt WB. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967;16:483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- 10.Kloetzel K. A suggestion for the prevention of severe clinical forms of Schistosomiasis mansoni. Bull WHO. 1967;37:686–687. [PMC free article] [PubMed] [Google Scholar]
- 11.Grimaud JA, Druget M, Peyrol S, Chevalier D. Collagen immunotyping in human liver: light and electron microscope study. J Histochem Cytol. 1980;28:1145–1151. doi: 10.1177/28.11.7000887. [DOI] [PubMed] [Google Scholar]
- 12.Engvall E, Perlman P. Enzyme linked immunosorbent assay: quantitative assay of IgE. Immunochemistry. 1971;8:871. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 13.Jürg U, Jennifer K, Xiao S, Marcel T, Burton H. Combination chemotherapy of schistosomiasis in laboratory studies and clinical trials. Antimicrob Agents Chemother. 2003;47:1487–1495. doi: 10.1128/AAC.47.5.1487-1495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Flamme CA, Patton EA, Pearce JE. Role of gamma interferon in the pathogenesis of severe schistosomiasis in interleukin–4–deficient mice. Infect Immun. 2001;69:7445–7452. doi: 10.1128/IAI.69.12.7445-7452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang BB, Jiao YW, Cai WM, Tao J, Liu RH. Influence of interferon gamma treatment on expression of TGF–beta1 and its receptors in liver fibrosis of mice with schistosomiasis japonica. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2004;22:340–343. [PubMed] [Google Scholar]
- 16.Henri S, Chevillard C, Mergani A, et al. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN is associated with protection against fibrosis and TNF with aggravation of disease. J Immunol. 2002;169:929–936. doi: 10.4049/jimmunol.169.2.929. [DOI] [PubMed] [Google Scholar]
- 17.Lins de Morais NC, Rodrigues de Souza J, Gomes de Melo W, et al. Studies on the production and regulation of interleukin, IL–13, IL–4 and interferon–g in human schistosomiasis mansoni. Mem Inst Oswaldo Cruz J. 2002;97(Suppl 1):113–114. doi: 10.1590/s0074-02762002000900023. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro de Jesus A, Araújo I, Bacellar O, et al. Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect Immun. 2000;68:2797–2803. doi: 10.1128/iai.68.5.2797-2803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naus WAC, Kimani G, Ouma HJ, et al. Development of antibody isotype responses to Schistosoma mansoni in an immunologically naive immigrant population: influence of infection duration, infection intensity, and host age. Infect Immun. 1999;67:3444–3451. doi: 10.1128/iai.67.7.3444-3451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Dam GJF, Stelma F, Gryseels B, et al. Antibody response patterns against Schistosoma mansoni in a recently exposed community in Senegal. J Infect Dis. 1996;173:1232–1241. doi: 10.1093/infdis/173.5.1232. [DOI] [PubMed] [Google Scholar]
- 21.Webster M, Roberts M, Fulford AJC, et al. Human IgE responses to r22.6 are associated with infection intensity rather than age per se, in a recently established focus of Schistosomiasis mansoni. Trop Med Int Health. 1998;3:318–326. doi: 10.1046/j.1365-3156.1998.00234.x. [DOI] [PubMed] [Google Scholar]
- 22.Capron M, Capron A. Immunoglobulin E and effector cells in schistosomiasis. Science. 1994;264:1876–1877. doi: 10.1126/science.8009216. [DOI] [PubMed] [Google Scholar]
- 23.Bahia–Oliveira LM, Simpson AJ, Alves–Oliveira LF, et al. Evidence that cellular immune responses to soluble and membrane associated antigens are independently regulated during human schistosomiasis mansoni. Parasite Immunol. 1996;18:53–63. doi: 10.1046/j.1365-3024.1996.d01-49.x. [DOI] [PubMed] [Google Scholar]
- 24.Biggelaar AHV, Grogan JL, Filie Y, et al. Chronic schistosomiasis: dendritic cells generated from patients can overcome antigen–specific T cell hyporesponsiveness. J Infect Dis. 2000;182:260–265. doi: 10.1086/315662. [DOI] [PubMed] [Google Scholar]
- 25.Colley DG, Sasser LE, Reed AM. PD–L2+ dendritic cells and PD–1+ CD4+ T cells in schistosomiasis correlate with morbidity. Parasite Immunol. 2005;27:45–53. doi: 10.1111/j.1365-3024.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 26.Cho Y, De Bruyn PPH. Passage of red blood cells through the sinusoidal wall of the spleen. Am J Anat. 1975:142–191. doi: 10.1002/aja.1001420107. [DOI] [PubMed] [Google Scholar]
- 27.West MB, Hill BG, Xuan YT, Bhatnagar A. Protein glutathiolation by nitric oxide: an intracellular mechanism regulating redox protein modification. FASEB J. 2006;20:1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]
- 28.Giovanna F, Stefano I, Luca P, et al. 8–Hydroxyquinoline derivatives as fluorescent sensors for magnesium in living cells. J Am Chem Soc. 2006;128:344–350. doi: 10.1021/ja056523u. [DOI] [PubMed] [Google Scholar]


