Abstract
Optic vesicle formation, transformation into an optic cup and integration with neighboring tissue are essential for normal eye formation, and involve the coordinated occurrence of complex cellular and molecular events. Perhaps not surprisingly, these complex phenomena have provided fertile ground for controversial and even contradictory results and conclusions. After presenting an overview of current knowledge of optic vesicle development, we will address conceptual and methodological issues that complicate research in this field. This will be done through a review of the pertinent literature, as well as by drawing on our own experience, gathered through recent studies of both intra- and extra-cellular regulation of optic vesicle development and patterning. Finally, and without attempting to be exhaustive, we will point out some important aspects of optic vesicle development that have not yet received enough attention.
Keywords: Epigenetic mechanisms, Extracellular signaling molecules, Eye development, Morphogenetic mechanisms, Lens induction, Optic cup, Optic vesicle, Pattern formation, Retina development, Retinal pigment epithelium, Transcription factors
I) Introduction
This article will present an overview of the complex cellular and molecular mechanisms underlying the formation of the optic vesicle (OV), the interactions of the OV with neighboring tissues, and its transformation into an optic cup (OC) containing three different but interconnected domains (neural retina, retinal pigment epithelium and optic stalk). These phenomena have attracted much attention since the early days of experimental embryology but, despite considerable progress, they remain incompletely understood, and even controversial. Space limitations make it impossible to cover the entire field, or to do justice to the many important contributions made by different laboratories over the years. Comprehensive reviews of the studies done before the year 2000 are available (e.g.,Chow and Lang, 2001; Jean et al., 1998; Zhang et al., 2002; Lupo et al., 2000) and more recent contributions have been partially reviewed (e.g., Bailey et al., 2004; Esteve and Bovolenta, 2006; Lovicu and McAvoy, 2005; Lupo et al., 2006; Sullivan et al., 2004; Wilson and Houart, 2004; Yang, 2004; Zaghloul et al., 2005, among others). We will only present here a brief summary of this material, as background for a discussion of problems of experimental design and interpretation that are at least partially responsible for controversies in this field. In a closing section, we will summarize important aspects of these phenomena that have not yet received much experimental analysis. The information summarized in this article has been derived from studies on embryos from several different experimental animal species, including amphibians, chick, mouse, and to a lesser extent rat and fish. Although interspecific differences are only considered in some detail in Section V-B, it is not unlikely that they could also be relevant to phenomena described in other parts of the article.
II) Structural Aspects of Optic Vesicle Development
The initial indication of optic vesicle (OV) formation is the appearance of symmetrical bilateral evaginations from the diencephalon, which slowly expand through the mesenchyme towards the surface ectoderm (Fig 1A). As contact between the OV and the ectoderm is established (Fig 1B), both tissues undergo complex (and temporally correlated) structural changes. The ectoderm thickens initially into a “lens placode” (Fig 1B), which invaginates into a vesicle that eventually closes and separates completely from the surface ectoderm (Fig 1C–D). The concomitant invagination of the OV is more complex, resulting in the formation of an optic cup (OC) that has a double wall, and is connected to the diencephalon by the optic stalk (Fig 1C–E). The invagination of the dorsal aspect of the optic vesicle (Fig 1C) generates an internal layer (the neural retina) and an external layer, the retinal pigment epithelium (RPE), whereas more ventrally the OV narrows considerably into the “choroid fissure” (Fig 1C’, D). The fissure closes completely in normal development, forming the optic nerve through which retinal ganglion cell grow axons towards the brain (Fig 1E); its abnormal persistence is known as “coloboma”.
Figure 1.
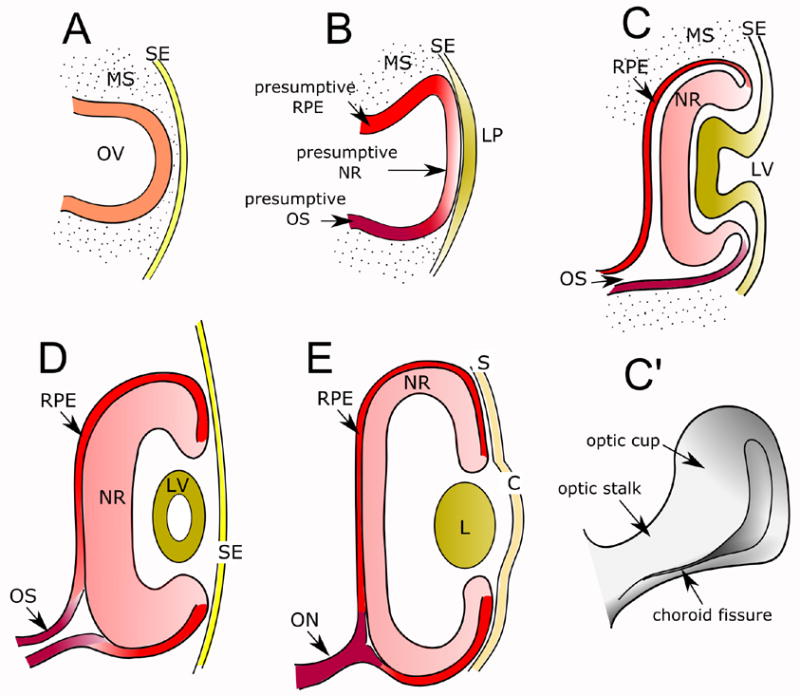
Schematic representation of vertebrate eye development. A. The optic vesicle forms as an evagination from the diencephalon. B. Upon contact with the surface ectoderm, the optic vesicle becomes patterned into presumptive RPE, neural retina and optic stalk; the surface ectoderm in turn forms the lens placode. C. The optic vesicle and lens placode invaginate, giving rise to the optic cup and the lens vesicle, respectively. C′. The ventral region of the invaginating optic vesicle forms the choroid fissure. D-E. Transition from early to mature optic cup. The lens vesicle loses its cavity and becomes a solid structure; the neural retina and the pigment epithelium become apposed, reducing ependymal cavity to a virtual space; the optic stalk gives rise to the optic nerve, and the surface ectoderm adjacent to the lens gives rise to the corneal epithelium. Abbreviations: C: Cornea; L: lens; LP: lens placode; LV: lens vesicle; MS: mesenchyme; NR: neural retina; ON: optic nerve; OS: optic stalk; OV: optic vesicle; RPE: retinal pigment epithelium; S: sclera; SE: surface ectoderm.
III) Inductive interactions during OV development
An extensive series of inductive tissue interactions must occur for the OV to appear, develop, and integrate with neighboring tissues. They actually begin well before the first morphological indications of OV development, as illustrated by the induction and regionalization of the neural plate by the “organizer” (Kessler and Melton, 1994; Streit and Stern, 1999), and the induction of an “eye field” within the anterior neural plate (Esteve and Bovolenta, 2006; Moore et al., 2004; Zuber et al., 2003). The eye field is located in the anterior neural plate, surrounded by telencephalic precursors and by cells that will form the hypothalamus (Esteve and Bovolenta, 2006). The precordal mesoderm plays a key role in determining the eye field and its subdivision into two separate domains, the future bilateral optic vesicles (Li et al., 1997). As the evaginating optic vesicles make contact with the mesenchyme and the ectoderm, they form a highly interactive system in which numerous consecutive, and frequently reciprocal inductive interactions take place. The specification of the neural retina and RPE domains within the OV appears to be determined by inductive signals originating in the surface ectoderm and in the mesenchyme, respectively (Fuhrmann et al., 2000; Vogel-Hopker et al., 2000; Zhao et al., 2001). In turn, inductive influences from the optic vesicle derivatives influence further development of the lens (Chow and Lang, 2001; Grainger et al., 1997; Lovicu and McAvoy, 2005). The dorso-ventral patterning of the optic cup is regulated by a balance between opposing signals originating in the neural tube and/or the optic stalk on one hand, and in the dorsal region of the optic cup on the other. The lens also influences the formation of the iris and ciliary body, specialized structures at the peripheral margin of the optic cup (Hyer, 2004). Several aspects of these interactions will be discussed in more detail below.
IV) Extracellular signaling molecules and transcription factors involved in OV induction and development
A) Extracellular signaling molecules
The secreted signaling molecules involved in the regulation of eye development belong to a “surprisingly restricted number of gene families” (Esteve and Bovolenta, 2006), including hedgehog (Hh), Wnt, transforming growth factor β (TGF-β), bone morphogenetic proteins (BMPs), and fibroblast growth factor (FGF). Many of these signaling molecules reappear at different stages of eye development, controlling different developmental events, as summarized in Table 1. FGF and the Wnt non-canonical pathway have been shown to regulate the morphogenetic movements of progenitor cells towards the eye field in Xenopus (Lee et al., 2006; Moody, 2004; Moore et al., 2004). The non- canonical branch of the Wnt signaling pathway subsequently ensures the cohesion of progenitor cells in the eye field (Cavodeassi et al., 2005), while its canonical/βcatenin branch has to be dowregulated or inhibited for the eye field to differentiate from the diencephalic region (reviewed by (Esteve and Bovolenta, 2006; Wilson and Houart, 2004). In addition, interplay between the non-canonical Wnt pathway and BMP appears to influence the establishment of a boundary between telencephalon and eye field (reviewed by Esteve and Bovolenta, 2006). Shisa, a recently identified protein, may play an important role in regulating Wnt and FGF signaling during the early events leading to optic vesicle formation (Filipe et al., 2006; Yamamoto et al., 2005). One of the factors involved in splitting the eye field is a secreted Nodal-related member of the TGF-β superfamily encoded by the Cyclops (Cyc) gene (Muller et al., 2000; Varga et al., 1999), perhaps acting through the induction of SHH expression (Chow and Lang, 2001; Ekker et al., 1995; Li et al., 1997; Macdonald et al., 1995; Marti and Bovolenta, 2002; Muller et al., 2000; Pera and Kessel, 1997; Wilson and Houart, 2004). SHH, in turn, contributes to the normal separation of the eye field into two optic vesicles by regulating its proximo-distal patterning (Chow and Lang, 2001; Ekker et al., 1995; Macdonald et al., 1995; Take-uchi et al., 2003; Wilson and Houart, 2004).
Table 1.
Extracellular molecules involved in eye development through early optic cup stages
BMPs: bone morphogenetic proteins; DAN: DAN domain family members; FGFs: fibroblast growth factors; RA: retinoic acid; SHH: sonic hedgehog; Wnts: members of the Wnt family.
As already mentioned, patterning of the OV neuroepithelium into neural retina (NR) and retinal pigment epithelium (RPE) depends on inductive signals from neighboring surface ectoderm and mesenchyme. FGF family members expressed in the surface ectoderm appear to induce neural retina formation (reviewed by Bharti et al., 2006; Chow and Lang, 2001; Martinez-Morales et al., 2004). In addition, upon contact with the surface ectoderm the prospective neural retina itself expresses FGF8 and FGF9, both of which play a role in defining the boundary between NR and RPE (Galy et al., 2002; Horsford et al., 2005; Vogel-Hopker et al., 2000; Zhao et al., 2001); among others.) Extraocular mesenchyme promotes RPE differentiation, on the other hand, possibly through an activin-like signal (Fuhrmann et al., 2000; Kagiyama et al., 2005; reviewed by Bharti et al., 2006; Chow and Lang, 2001; Martinez-Morales et al., 2004). BMP7 expression within the prospective RPE domain helps to maintain the identity of this tissue by antagonizing possible neuralizing effects of FGF (Vogel-Hopker et al., 2000). SHH is also required for the dorso-ventral and central-to-periphery patterning of the optic cup at a later stage (Zhang and Yang, 2001; reviewed by Peters, 2002). BMPs and retinoic acid (RAc) have also been considered important players in dorso-ventral patterning regulation; their specific roles remain controversial, however, and will be discussed below.
B) Transcription factors
Transcription factors are also frequently involved in the regulation of more than one aspect of eye development, as summarized in Table 2 (Bailey et al., 2004; Chow and Lang, 2001; Esteve and Bovolenta, 2006). Rx, Pax6 and Otx2 have been shown to play a role in the morphogenetic movements leading to eye field formation, probably under the influence of extra-cellular signaling molecules (Esteve and Bovolenta, 2006). In addition, the coordinated expression of Rx, Pax6, Six3, Lhx2, Six6/Optx2, ET and tll appears essential for the specification of the eye field (Bailey et al., 2004; Chow and Lang, 2001; Viczian et al., 2006; Wilson and Houart, 2004; Zuber et al., 2003). Although knockout and mis-expression experiments have shown that some of these genes can regulate each other’s expression, their epistatic relationships are not clear (Chow and Lang, 2001; Zuber et al., 2003). Interestingly, early eye development also appears to be influenced by transcription factors that are not expressed in the eye field, such as Hes1 and Otx2, which may act indirectly by regulating forebrain development (Bailey et al., 2004; Chow and Lang, 2001).
Table 2.
Transcription factors involved in eye development through early optic cup stages
Several transcription factors have also been shown to influence optic vesicle formation and/or evagination, which fail to occur in Rx mouse mutants and are abnormal in Pax6 mouse mutants through still unknown mechanisms (Bailey et al., 2004; Chow and Lang, 2001). Analysis of Rx3 mutants in medaka and zebrafish suggested that it may affect optic vesicle evagination by influencing active cell migration from the eye field (Loosli et al., 2003; Loosli et al., 2001; Rembold et al., 2006). In addition, inhibition of tll has also been shown to interfere with optic vesicle evagination in Xenopus (Hollemann et al., 1998). Neuroepithelial cells of the early optic vesicle co-express Rx, Pax6, Hes1, Otx2, Lhx2, Six3 and Six9 while they are still competent to originate optic stalk, neural retina and RPE (reviewed by Chow and Lang, 2001; Martinez-Morales et al., 2004). The subsequent specification of these OV derivatives is accompanied by differential expression of these and other transcription factors: Pax2 and Vax in the prospective optic stalk; Pax6, Rx, Lhx2 and Chx10 in the prospective neural retina, and Pax6, Otx2 and Mitf in the prospective RPE (Bharti et al., 2006; Chow and Lang, 2001; Martinez-Morales et al., 2004). Reciprocal transcriptional repression between transcription factors may contribute to establishing boundaries between developing territories (eg, Pax6 and Pax2 for neural retina and optic stalk, Chx10 and Mitf for neural retina and RPE (Canto-Soler and Adler, 2006; Horsford et al., 2005; Schwarz et al., 2000).
Pax6, Hes1, and Lhx2 are necessary for proper growth of the optic vesicle and its transformation into an optic cup. As discussed below in some detail, Pax6 downregulation in the optic vesicle neuroepithelium affects the survival of optic vesicle cells and the transformation of the OV into a normal optic cup (Canto-Soler and Adler, 2006). Similarly, Lhx2 knockout mice develop optic vesicles, but optic cup and lens formation fail to occur (Porter et al., 1997; reviewed by Chow and Lang, 2001). The phenotype of Hes1 mutant mice varies from a reduced lens accompanied by a smaller than normal optic cup, to the complete absence of the lens with an arrested optic vesicle (Lee et al., 2005; Tomita et al., 1996).
In the optic cup, interactions between Pax6, Pax2, cVax and Tbx5 mediate dorso-ventral patterning of the neural retina (Canto-Soler and Adler, 2006; Leconte et al., 2004; reviewed by Chow and Lang, 2001; Peters, 2002). In addition, Pax6 activity is required for the establishment and maintenance of dorsal and naso-temporal characteristics (Baumer et al., 2002). Other transcription factors also involved in naso-temporal patterning of the neural retina are BF-1/Foxg1, BF-2/Foxd2, SOHo1 and GH6 (Takahashi et al., 2003; Yuasa et al., 1996; reviewed by Chow and Lang, 2001; Peters, 2002).
V) Complexities, ambiguities and complications in OV research
Lens induction, one of the first developmental phenomena to be studied experimentally, has provided fertile ground for controversial and even contradictory conclusions, particularly regarding whether the optic vesicle is essential for lens development to start and/or proceed (Lovicu and McAvoy, 2005; Sullivan et al., 2004). The diverging results obtained by different investigators were eventually ascribed to differences in methodology, in the selection of experimental animals, and/or in the developmental stages and end points used to analyze these complex phenomena. Perhaps not surprisingly, equivalent discrepancies and controversies have emerged more recently among experimental studies of other aspects of early eye development that, like lens induction, are complex multi-step processes, rather than simple one-step phenomena. These complications are the rule rather than the exception, and should in fact be expected and anticipated by researchers in this field. We will illustrate the point by describing, in the context of the appropriate literature, the complexities that we encountered in designing and interpreting two recent studies of micro-environmental factors and transcriptional regulators involved in optic cup development (Adler and Belecky-Adams, 2002; Canto-Soler and Adler, 2006).
A) The complexity of extra-cellular signaling systems in optic cup development
The dorsal and ventral regions of the optic cup show many differences since early embryonic stages. They include: i) structural differences (e.g., the dorsal retina expands faster than the ventral retina, and the latter forms a choroid fissure (Koshiba-Takeuchi et al., 2000); ii) molecular differences (e.g., they express different transcription factors (Barbieri et al., 1999; Koshiba-Takeuchi et al., 2000; Macdonald et al., 1995; Ohsaki et al., 1999; Schulte et al., 1999; Torres et al., 1996), retinoic acid synthesizing enzymes (McCaffery et al., 1999; Mey et al., 2001; Suzuki et al., 2000), ephrins and ephrin receptors (Braisted et al., 1997; Holash and Pasquale, 1995; Holash et al., 1997; Marcus et al., 1996) and BMPs (Belecky-Adams and Adler, 2001); and iii) functional differences (e.g., they project to different brain regions (Braisted et al., 1997; Thanos and Mey, 2001), and respond differently to various regulatory molecules (Hyatt and Dowling, 1997; Marsh-Armstrong et al., 1994; Zhang and Yang, 2001). There has been considerable interest, therefore, on the signals that influence the dorso-ventral patterning of the retina.
Retinoic acid and sonic hedgehog have been generally recognized as regulators of ventral retinal development (Hyatt et al., 1996a; Hyatt et al., 1996b; Kastner et al., 1994; Marsh-Armstrong et al., 1994; Zhang and Yang, 2001), whereas the BMPs (and particularly BMP4) have been described predominantly as regulators of dorsal retinal development (Koshiba-Takeuchi et al., 2000; Zhang and Yang, 2001; reviewed by Peters, 2002). Their roles, however, are currently under revision. BMP4 is restricted to the dorsal region of the optic cup, and its experimental over-expression has dorsalizing effects upon the ventral retina (Koshiba-Takeuchi et al., 2000; Sasagawa et al., 2002; Trousse et al., 2001). During normal development, the ventral retina is apparently protected from these effects by ventroptin, a BMP inhibitor (Koshiba-Takeuchi et al., 2000; Sakuta et al., 2001), and by sonic hedgehog (SHH), a ventralizing agent (Zhang and Yang, 2001). These results led to the formulation of a model proposing that, as in the case of the ventro-dorsal patterning of the spinal cord (reviewed by Jessell, 2000), ventral Shh signals and dorsal BMP4 signals act antagonistically, establishing and maintaining distinct ventral and dorsal eye compartments respectively (Peters, 2002; Yang, 2004; Zhang and Yang, 2001). Against this background, it was surprising to find that inhibition of BMP signaling by noggin over-expression caused extensive abnormalities of ventral eye structures, whose severity varied considerably as a function of the stage of the embryo at treatment onset (Adler and Belecky-Adams, 2002). Noggin overexpression at optic vesicle stages resulted in microphthalmia with concomitant disruption of the developing neural retina, RPE and lens, whereas the same treatment started at optic cup stages did not affect the size and general organization of the eye, but caused colobomas, absence of the pecten, transdifferentiation of the ventral RPE into neuroepithelium-like tissue, ectopic expression of optic stalk markers in the ventral retina and RPE, and ectopic growth of optic nerve fibers towards the lens (Adler and Belecky-Adams, 2002). Similar results were obtained with a dominant negative BMP receptor (Adler and Belecky-Adams, 2002), by overexpression of DRM-gremlin another BMP inhibitor (Huillard et al., 2005), an in BMP7-deficient mice (Morcillo et al., 2006). These results imply that endogenous BMPs regulate ventral optic cup development despite their co-existence with a variety of BMP inhibitors, including follistatin, follistatin-like protein (flik), DAN, chordin, noggin and ventroptin (Belecky-Adams and Adler, 2001; Belecky-Adams et al., 1999; Eimon and Harland, 2001; Ogita et al., 2001; Sakuta et al., 2001). The data also suggest that BMP agonists and antagonists must exist in a precisely fine-tuned equilibrium within optic cup tissues. It must be noted also that the developing optic cup and adjacent tissues express several BMPs in addition to BMP4 (Belecky-Adams and Adler, 2001); BMP7 is expressed near the optic stalk and in the ventral pigment epithelium, for example, and is therefore strategically located to influence the ventral retina (Belecky-Adams and Adler, 2001; Vogel-Hopker et al., 2000). BMP receptors IA and IB, moreover, are predominantly if not exclusively localized to the ventral retina and optic stalk at early developmental stages (Belecky-Adams and Adler, 2001). Taken together, these findings disclose three layers of complexity that are shared by other signaling systems in the developing optic cup, and are illustrated in Figure 2. They are: i) families of growth factors are frequently represented by many members in the optic cup and adjacent tissues, ii) they frequently coexist with inhibitors, and iii) tissues that are morphologically homogeneous show heterogeneity in the distribution of signaling molecules and their inhibitors and receptors. Representative examples of other families of growth factors showing similar complexities are FGFs and their respective high and low affinity receptors (Fig. 2B), (Francisco-Morcillo et al., 2005; Hicks, 1998; Itoh and Ornitz, 2004; Kurose et al., 2005; Lovicu and McAvoy, 2005; Ornitz and Itoh, 2001; Vogel-Hopker et al., 2000; Yamashita et al., 2000) and the Wnts (Fig. 2C), (Fokina and Frolova, 2006; Fuhrmann et al., 2003; Jin et al., 2002; Liu et al., 2003; Van Raay and Vetter, 2004).
Figure 2.

Schematic representation of the distribution of members of several families of extracellular signaling systems in the developing optic cup. The data shown corresponds predominantly to in situ hybridization results. Gradients of color represent the superposition of expression domains of different molecules, rather than gradients of expression of individual molecules (for the sake of clarity, only the region corresponding to the highest level of expression has been represented for those molecules whose pattern of expression follows high-to-low gradients). A. BMPs, its receptors, antagonists and inhibitors (modified and up-dated from Belecky-Adams, unpublished). B. Wnts, its receptors and antagonists (modified and up-dated from Van Raay and Vetter, 2004). C. FGFs, its receptors and antagonists. Abbreviations: BMP: bone morphogenetic proteins; CRF: cysteine-rich FGF receptors; Dan: members of the Dan protein family; FGF: fibroblast growth factors; Fz: frizzled receptors; Sfrp: secreted frizzled related proteins; Spry: sprouty protein; Wnt: members of the Wnt family.
An additional complication for the interpretation of noggin overexpression experiments was that at least some of its effects on ventral retina could be indirect, resulting from primary effects on other tissues and/or by changes in the expression of other signaling molecules. Such indirect effects would not be without precedent. For example, lens induction was absent in BMP4 homozygous null mutant embryos, and could be rescued by exogenous BMP4 protein (Furuta and Hogan, 1998), suggesting that BMP4 has a direct effect on lens induction. However, the same authors found that BMP4 failed to induce lens when it was applied to ectoderm in the absence of optic vesicle, leading to the conclusion that BMP4 is in fact required within the optic vesicle itself to determine its lens-inducing activity. Our finding that noggin over-expression in the retina induced alterations in lens development (Belecky-Adams et al., 2002) raised the possibility that such lens alterations could affect its capacity to interact with the retina, particularly considering the complexity of the effects of BMP4 and BMP7 on lens induction (Dudley et al., 1995; Jena et al., 1997; Karsenty et al., 1996; Luo et al., 1995; Solursh et al., 1996; Wawersik et al., 1999). Noggin-induced mis-direction of ganglion cell axons away from the optic stalk also appeared to be indirect, since it was accompanied by an intraretinal expansion of the domain of expression of netrin (Adler and Belecky-Adams, 2002), an axonal guidance molecule that normally attracts ganglion cell axons into the optic nerve (de la Torre et al., 1997; Deiner et al., 1997; Livesey and Hunt, 1997; Petrausch et al., 2000; Sugimoto et al., 2001). Similarly, the apparent transdifferentiation of ventral eye tissues induced by noggin over-expression was accompanied by the upregulation of FGF-8 (Adler and Belecky-Adams, 2002) which can induce RPE transdifferentiation (Vogel-Hopker et al., 2000); it is noteworthy that these authors suggested that BMP7, produced by the pigment epithelium, prevents FGF8 from inducing RPE transdifferentiation during normal eye development. Noggin-treated retinas also showed upregulation of ALDH6, a retinoic acid-synthesizing enzyme, suggesting that retinoic acid may also have contributed to the observed changes in the ventral retina. The notion that endogenous retinoic acid plays a critical role in dorso-ventral retinal patterning is supported by i) localization studies in several species (Drager et al., 2001; Duester et al., 2003; Luo et al., 2006; Matt et al., 2005; Peters and Cepko, 2002; Wagner et al., 2000), ii) experiments showing that treatment of the zebrafish eye with retinoic acid leads to duplication of the ventral retina (Hyatt et al., 1992), and iii) the finding that the inhibition of RAc synthesis with citral has the opposite effects (Marsh-Armstrong et al., 1994). On the other hand, loss-of-function experiments in the chick have suggested that RAc indeed plays a role in regulating the expression of dorso-ventral topographic guidance molecules in the retina, but does so without altering the expression of transcription factors involved in dorso-ventral patterning, such as Tbx5 or Vax (Sen et al., 2005). Equivalent results have been recently reported in the mouse (Matt et al., 2005; Molotkov et al., 2006). Obviously, indirect effects of signaling molecules are far from exceptional in a highly integrated and interactive system like the developing eye.
B) The complexity of transcription factor effects: role of Pax6 in optic cup development
Heterozygous mutations in the Pax6 homeobox gene cause human aniridia and Peter’s anomaly (Glaser et al., 1992; Hanson et al., 1994; Jordan et al., 1992), and the small eye phenotype in mice and rats (Hill et al., 1991; Matsuo et al., 1993). Anophthalmia can result either from loss-of-function Pax6 mutations (Glaser et al., 1994; Hill et al., 1991) or from Pax6 overexpression, suggesting dosage-dependent effects (Schedl et al., 1996). The elucidation of the underlying mechanisms has been challenging due to the reciprocal inductive interactions between the OV and the lens ectoderm, and to the complex patterns of Pax6 expression in both structures (Belecky-Adams et al., 1997; Grindley et al., 1995; Li et al., 1994; Walther and Gruss, 1991; reviewed by Chow and Lang, 2001). Until recently, there was general (but not universal) acceptance of the notions that i) Pax6 is not essential for optic vesicle formation or for the establishment of the NR and RPE domains; ii) the primary defect leading to the small eye phenotype is the failure of the surface ectoderm to form a lens placode, which in turn leads to degeneration of the OV; and iii) lens development requires Pax6 activity in the prospective lens ectoderm but not in the optic vesicle (reviewed by Ashery-Padan and Gruss, 2001; Lang, 2004; Mathers and Jamrich, 2000; Ogino and Yasuda, 2000) This view will be referred henceforth as the "Pax6/lens" model. Surprisingly, experiments in which anti-Pax6 morpholinos were electroporated into the chick embryo optic vesicle before the onset of lens placode formation (Canto-Soler and Adler, 2006) showed that i) early eye development requires cell-autonomous Pax6 function not only in the lens but also in the optic vesicle; ii) a small-eye like phenotype can occur even when Pax6 is normally expressed in the surface ectoderm; iii) Pax6 expression in the OV is necessary for the survival of retinal progenitor cells and for the specification of NR and RPE domains; iv) Pax6 expression in the optic vesicle is also necessary for normal lens development during a critical developmental stage; and v) Pax6 expression in the lens is necessary, but not sufficient for normal less development. We will refer to this view as the “Pax6/lens-OV” model. Although these discrepancies have not yet been fully resolved, they provide useful insights into the factors that complicate experimental analysis of early eye development.
Possible differences between chick and mouse/rat embryos could contribute to the discrepancies, since the Pax6/lens-OV model was supported by experiments with chick embryos (Canto-Soler and Adler, 2006; Reza and Yasuda, 2004), while supportive evidence for the Pax6/lens model derived from mouse and rat studies (Ashery-Padan et al., 2000; Davis-Silberman et al., 2005; Fujiwara et al., 1994; Hill et al., 1991; Hogan et al., 1988; Zhang et al., 2000). Such possible inter-specific differences are unlikely to be the only explanation, however, because it has been reported that, prior to the time of lens placode formation, Sey/Sey mouse OVs already are abnormally broad, and fail to constrict proximally (Grindley et al., 1995); analysis of Pax6 chimeric mice, moreover, suggested that Pax6 is required in the OV for maintenance of contact with the overlying lens epithelium (Collinson et al., 2000). The very dynamic nature of the optic vesicle and surrounding tissues is another likely contributor to differences in experimental outcomes between studies, or even within one same study. In our experiments, for example, Pax6 downregulation was already detectable in the OV 10 h after anti-Pax6 morpholino electroporation, and was accompanied by a significant increase in the death of OV cells; the magnitude and phenotypic consequences of these changes, however, were dramatically different depending on the embryonic stage at treatment onset (Canto-Soler and Adler, 2006). When morpholinos were electroporated at Hamburger Hamilton (HH) stage 10, there was no optic cup formation, and lens development was abortive despite normal Pax6 expression in the lens epithelium. On the other hand, treatment at HH stage 11 resulted in structurally normal lens and optic cup, although the latter showed abnormal expression domains for several transcription factors. What makes these differences even more striking is that the time interval between stages 10 and 11 is only 7 hours. An important corollary of these findings is that, if we had done our experiments only at HH stage 11, they would have yielded results consistent with the Pax6/lens model, rather than with the Pax6/lens-OV model. Crucial for the outcome of our experiments was the high temporal resolution offered by the combination of electroporation techniques and morpholino oligonucleotides, whose antisense activity starts very shortly after treatment. The timing of this experimental treatment may be different from that of other approaches, such as cre-lox dependent Pax6 inactivation (Ashery-Padan et al., 2000; Davis-Silberman et al., 2005) or expression of dominant negative constructs (Reza and Yasuda, 2004).
Some of the discrepancies between the Pax6/lens-OV and Pax6/lens models may only be apparent, and may result from the "end points" used to evaluate experimental outcomes. The notion that Pax6 expression in the lens epithelium is necessary for normal lens development has been well supported by experiments using conditional knockouts and chimeras in the mouse (Ashery-Padan et al., 2000; Collinson et al., 2000; Quinn et al., 1996). On the other hand, the notion that Pax6 expression in the lens is also sufficient for lens development is predominantly based on recombination experiments using tissues isolated from wild type and small eye (rSey/rSey) rats (Fujiwara et al., 1994). These experiments showed that the onset of lens formation depends upon the genotype of the surface ectoderm, and is independent of the genotype of the optic vesicle. However, the recombination experiments were not long enough to allow evaluating the degree of differentiation reached by the lens in each case, and it is therefore unknown whether lens development would have become arrested in the presence of Sey/Sey optic vesicles, as it did in the chick studies when PAX6 was downregulated in the optic vesicle (Canto-Soler and Adler, 2006; Reza and Yasuda, 2004). This possibility is not unlikely given that lens development appears to occur through at least 4 distinct stages, which may be regulated by different mechanisms (Grainger et al., 1992; Grainger et al., 1988; Grainger et al., 1997; Henry and Grainger, 1987; Saha et al., 1989). The dynamic nature of the lens epithelium may also explain controversies regarding whether the lens is needed for the invagination of the optic vesicle and the formation of the neural retina. Thus, cre-lox inactivation of Pax6 in the lens led to the conclusion that the lens itself is not needed for optic cup invagination or the appearance of a retina (although it is for its placement (Ashery-Padan et al., 2000) whereas more recent experiments confirmed that the lens itself is not necessary, but showed that the optic vesicle neuroepithelium does require a temporally specific association with pre-lens ectoderm in order to undergo morphogenesis into an optic cup (Hyer et al., 2003; Khosrowshahian et al., 2005).
VI) Aspects of OV development that have not received extensive attention
While no aspect of optic vesicle formation and development has been fully explained, progress has been quite substantial in many areas, and the existing momentum in their investigation is reason for optimism that progress will continue at a fast pace. On the other hand, there are several aspects of optic vesicle development that, despite their importance, have received relatively little attention. Without attempting to be comprehensive, we will close this article with an overview of two such areas.
1) Morphogenetic mechanisms
The invagination of the optic cup and the lens vesicle offers a striking example of precisely coordinated (and complex) morphogenetic phenomena. The regulation of both phenomena by micro-environmental factors has been studied. Many aspects of lens morphogenesis are profoundly influenced by micro-environmental factors, as shown by a series of elegant lens vesicle transplantation experiments in the chick embryo (Coulombre and Coulombre, 1963; reviewed by Lovicu and McAvoy, 2005). More recent experiments from several laboratories have shown that micro-environmental influences can also determine whether optic cup formation does or does not occur; they include the pre-lens ectoderm (Hyer et al., 2003), and retinoic acid (Matt et al., 2005; Mic et al., 2004; Molotkov et al., 2006). Considerable efforts have also been devoted to the analysis of the mechanisms of lens invagination, including possible complex changes in cell adhesion, cell shape, cell proliferation, cell death, and/or extracellular matrix molecules (reviewed by Menko et al., 1998; Zelenka, 2004). There has been much less work devoted to similar phenomena in the optic cup, beyond the demonstration that optic cup formation involves changes in cell shape accompanied by apparent increases in the number of microtubules (during cell elongation) and microfilaments (during apical cell constriction) (Brady and Hilfer, 1982; Schook, 1980; Svoboda and O’Shea, 1984; Svoboda and O’Shea, 1987). This is particularly surprising in view of the frequency with which optic cup morphogenesis results in abnormal defects of choroid fissure closure known as coloboma (Gregory-Evans et al., 2004; Onwochei et al., 2000), which can be caused by genetic factors such as mutations/deletions in PAX2 (reviewed by Dressler and Woolf, 1999), VAX (Hallonet et al., 1999), PITX2 (Gage et al., 1999), and SOX10 (Bondurand et al., 1999), and/or from changes in extracellular signaling molecules including SHH overexpression (Zhang and Yang, 2001), and decreases in the availability of retinoic acid (Stull and Wikler, 2000) or the BMPs (Adler and Belecky-Adams, 2002). It appears that the morphogenesis of the optic cup provides fertile ground for new investigations.
2) Epigenetic controls
Epigenetic mechanisms of gene expression control, including RNA-associated silencing, DNA methylation and histone modification, are being increasingly recognized as playing key roles in the control of normal embryonic development in a variety of tissues and species (reviewed by Bartel, 2004; Finnegan and Matzke, 2003; Grewal and Rice, 2004; Kloosterman and Plasterk, 2006; Lapidot, 2006; Song and Tuan, 2006; Werner, 2005 ). Examples in the nervous system include control of the timing of cell differentiation, the neuron versus glia fate choice of progenitor cells, neuronal plasticity, and dendritic spine development, among others (Abrahante et al., 2003; Conaco et al., 2006; Lin et al., 2003; Schratt et al., 2006; Vo et al., 2005; reviewed by Hsieh and Gage, 2004; Hsieh and Gage, 2005; Song and Tuan, 2006). In contrast, there is only a very limited body of literature dealing with these mechanisms in the developing eye. The timing of bHLH transcription factor function during retinal cell differentiation can be regulated post-translationally, and there seems to be a correlation between chromatin modifications and binding of these transcription factors (Moore et al., 2002; Skowronska-Krawczyk et al., 2004). More recently, several miRNAs, as well as natural antisense transcripts (NATs) for transcription factors such as Pax6, Pax2, Six3, Six6, Otx2, Crx, Rax and Vax, have been identified in vertebrate ocular tissue and found to exhibit distinct patterns of tissue and cell type distribution (Alfano et al., 2005; Frederikse et al., 2006; Ryan et al., 2006). In Drosophila, moreover, miRNA7 has been shown to promote photoreceptor differentiation (Li and Carthew, 2005). Despite these important but isolated findings, there is not yet a clear understanding of the role of epigenetic mechanisms in eye development. The paucity of information is even more conspicuous in the case of OV development; to the best of our knowledge, only two publications directly address these mechanisms. One of them is a description of the expression and function of Xenopus BMD3, a protein that specifically binds to methylated DNA, is highly expressed in the prospective eye region, and can influence early aspects of eye development (Iwano et al., 2004). In the other, the capacity of the Vax2 protein to repress Pax6 expression during the differentiation of the OV neuroepithelium was shown to be regulated by its phosphorilation/dephosphorilation, which in turn controls its translocation from the cytoplasm to the nucleus (Kim and Lemke, 2006). Considering the current pace of progress in understanding the significance of epigenetic mechanisms in normal development in general, it is to be expected that the investigation of their role in optic vesicle development will accelerate significantly in the near future.
Acknowledgments
Work in the authors laboratory has been supported by NIH grants EY 04859 and Core Grant EY 1765, by an unrestricted departmental grant from Research to Prevent Blindness, Inc (New York, New York), by the Knights of Templar, and by a contribution from the William Weiss Endowment for Research. RA is the Arnall Patz Distinguished Professor of Ophthalmology. The authors are grateful to Dr Teri Belecky-Adams for the figure used as a basis for Fig 2, and to Ms Jane Cione for secretarial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahante JE, Daul AL, Li M, Volk ML, Tennessen JM, Miller EA, Rougvie AE. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev Cell. 2003;4:625–37. doi: 10.1016/s1534-5807(03)00127-8. [DOI] [PubMed] [Google Scholar]
- Adler R, Belecky-Adams TL. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development. 2002;129:3161–71. doi: 10.1242/dev.129.13.3161. [DOI] [PubMed] [Google Scholar]
- Alfano G, Vitiello C, Caccioppoli C, Caramico T, Carola A, Szego MJ, McInnes RR, Auricchio A, Banfi S. Natural antisense transcripts associated with genes involved in eye development. Hum Mol Genet. 2005;14:913–23. doi: 10.1093/hmg/ddi084. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Curr Opin Cell Biol. 2001;13:706–14. doi: 10.1016/s0955-0674(00)00274-x. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–11. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TJ, El-Hodiri H, Zhang L, Shah R, Mathers PH, Jamrich M. Regulation of vertebrate eye development by Rx genes. Int J Dev Biol. 2004;48:761–70. doi: 10.1387/ijdb.041878tb. [DOI] [PubMed] [Google Scholar]
- Barbieri AM, Lupo G, Bulfone A, Andreazzoli M, Mariani M, Fougerousse F, Consalez GG, Borsani G, Beckmann JS, Barsacchi G, Ballabio A, Banfi S. A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc Natl Acad Sci U S A. 1999;96:10729–34. doi: 10.1073/pnas.96.19.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Baumer N, Marquardt T, Stoykova A, Ashery-Padan R, Chowdhury K, Gruss P. Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development. 2002;129:4535–45. doi: 10.1242/dev.129.19.4535. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams T, Adler R. Developmental expression patterns of bone morphogenetic proteins, receptors, and binding proteins in the chick retina. J Comp Neurol. 2001;430:562–72. [PubMed] [Google Scholar]
- Belecky-Adams T, Tomarev S, Li HS, Ploder L, McInnes RR, Sundin O, Adler R. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Invest Ophthalmol Vis Sci. 1997;38:1293–303. [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Scheurer D, Adler R. Activin family members in the developing chick retina: expression patterns, protein distribution, and in vitro effects. Dev Biol. 1999;210:107–23. doi: 10.1006/dbio.1999.9268. [DOI] [PubMed] [Google Scholar]
- Bharti K, Nguyen MT, Skuntz S, Bertuzzi S, Arnheiter H. The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. 2006;19:380–94. doi: 10.1111/j.1600-0749.2006.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N, Kuhlbrodt K, Pingault V, Enderich J, Sajus M, Tommerup N, Warburg M, Hennekam RC, Read AP, Wegner M, Goossens M. A molecular analysis of the yemenite deaf-blind hypopigmentation syndrome: SOX10 dysfunction causes different neurocristopathies. Hum Mol Genet. 1999;8:1785–9. doi: 10.1093/hmg/8.9.1785. [DOI] [PubMed] [Google Scholar]
- Brady RC, Hilfer SR. Optic cup formation: a calcium-regulated process. Proc Natl Acad Sci U S A. 1982;79:5587–91. doi: 10.1073/pnas.79.18.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisted JE, McLaughlin T, Wang HU, Friedman GC, Anderson DJ, O’Leary DD. Graded and lamina-specific distributions of ligands of EphB receptor tyrosine kinases in the developing retinotectal system. Dev Biol. 1997;191:14–28. doi: 10.1006/dbio.1997.8706. [DOI] [PubMed] [Google Scholar]
- Canto-Soler MV, Adler R. Optic cup and lens development requires Pax6 expression in the early optic vesicle during a narrow time window. Dev Biol. 2006;294:119–32. doi: 10.1016/j.ydbio.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Cavodeassi F, Carreira-Barbosa F, Young RM, Concha ML, Allende ML, Houart C, Tada M, Wilson SW. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron. 2005;47:43–56. doi: 10.1016/j.neuron.2005.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–96. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Hill RE, West JD. Different roles for Pax6 in the optic vesicle and facial epithelium mediate early morphogenesis of the murine eye. Development. 2000;127:945–56. doi: 10.1242/dev.127.5.945. [DOI] [PubMed] [Google Scholar]
- Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–7. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Lens Development: Fiber Elongation and Lens Orientation. Science. 1963;142:1489–90. doi: 10.1126/science.142.3598.1489. [DOI] [PubMed] [Google Scholar]
- Davis-Silberman N, Kalich T, Oron-Karni V, Marquardt T, Kroeber M, Tamm ER, Ashery-Padan R. Genetic dissection of Pax6 dosage requirements in the developing mouse eye. Hum Mol Genet. 2005;14:2265–76. doi: 10.1093/hmg/ddi231. [DOI] [PubMed] [Google Scholar]
- de la Torre JR, Hopker VH, Ming GL, Poo MM, Tessier-Lavigne M, Hemmati-Brivanlou A, Holt CE. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–24. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–89. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Drager UC, Li H, Wagner E, McCaffery P. Retinoic acid synthesis and breakdown in the developing mouse retina. Prog Brain Res. 2001;131:579–87. doi: 10.1016/s0079-6123(01)31045-2. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Woolf AS. Pax2 in development and renal disease. Int J Dev Biol. 1999;43:463–8. [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Duester G, Mic FA, Molotkov A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact. 2003;143–144:201–10. doi: 10.1016/s0009-2797(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Harland RM. Xenopus Dan, a member of the Dan gene family of BMP antagonists, is expressed in derivatives of the cranial and trunk neural crest. Mech Dev. 2001;107:187–9. doi: 10.1016/s0925-4773(01)00462-2. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–55. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Esteve P, Bovolenta P. Secreted inducers in vertebrate eye development: more functions for old morphogens. Curr Opin Neurobiol. 2006;16:13–9. doi: 10.1016/j.conb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Filipe M, Goncalves L, Bento M, Silva AC, Belo JA. Comparative expression of mouse and chicken Shisa homologues during early development. Dev Dyn. 2006;235:2567–73. doi: 10.1002/dvdy.20862. [DOI] [PubMed] [Google Scholar]
- Finnegan EJ, Matzke MA. The small RNA world. J Cell Sci. 2003;116:4689–93. doi: 10.1242/jcs.00838. [DOI] [PubMed] [Google Scholar]
- Fokina VM, Frolova EI. Expression patterns of Wnt genes during development of an anterior part of the chicken eye. Dev Dyn. 2006;235:496–505. doi: 10.1002/dvdy.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco-Morcillo J, Sanchez-Calderon H, Kawakami Y, Belmonte JC, Hidalgo-Sanchez M, Martin-Partido G. Expression of Fgf19 in the developing chick eye. Brain Res Dev Brain Res. 2005;156:104–9. doi: 10.1016/j.devbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Frederikse PH, Donnelly R, Partyka LM. miRNA and Dicer in the mammalian lens: expression of brain-specific miRNAs in the lens. Histochem Cell Biol. 2006;126:1–8. doi: 10.1007/s00418-005-0139-0. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Stark MR, Heller S. Expression of Frizzled genes in the developing chick eye. Gene Expr Patterns. 2003;3:659–62. doi: 10.1016/s1567-133x(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Uchida T, Osumi-Yamashita N, Eto K. Uchida rat (rSey): a new mutant rat with craniofacial abnormalities resembling those of the mouse Sey mutant. Differentiation. 1994;57:31–8. doi: 10.1046/j.1432-0436.1994.5710031.x. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–75. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–51. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Galy A, Neron B, Planque N, Saule S, Eychene A. Activated MAPK/ERK kinase (MEK-1) induces transdifferentiation of pigmented epithelium into neural retina. Dev Biol. 2002;248:251–64. doi: 10.1006/dbio.2002.0736. [DOI] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994;7:463–71. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2:232–9. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- Grainger RM, Henry JJ, Saha MS, Servetnick M. Recent progress on the mechanisms of embryonic lens formation. Eye. 1992;6 ( Pt 2):117–22. doi: 10.1038/eye.1992.26. [DOI] [PubMed] [Google Scholar]
- Grainger RM, Herry JJ, Henderson RA. Reinvestigation of the role of the optic vesicle in embryonic lens induction. Development. 1988;102:517–26. doi: 10.1242/dev.102.3.517. [DOI] [PubMed] [Google Scholar]
- Grainger RM, Mannion JE, Cook TL, Jr, Zygar CA. Defining intermediate stages in cell determination: acquisition of a lens-forming bias in head ectoderm during lens determination. Dev Genet. 1997;20:246–57. doi: 10.1002/(SICI)1520-6408(1997)20:3<246::AID-DVG7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gregory-Evans CY, Williams MJ, Halford S, Gregory-Evans K. Ocular coloboma: a reassessment in the age of molecular neuroscience. J Med Genet. 2004;41:881–91. doi: 10.1136/jmg.2004.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Rice JC. Regulation of heterochromatin by histone methylation and small RNAs. Curr Opin Cell Biol. 2004;16:230–8. doi: 10.1016/j.ceb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–42. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 1999;13:3106–14. doi: 10.1101/gad.13.23.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson IM, Fletcher JM, Jordan T, Brown A, Taylor D, Adams RJ, Punnett HH, van Heyningen V. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters’ anomaly. Nat Genet. 1994;6:168–73. doi: 10.1038/ng0294-168. [DOI] [PubMed] [Google Scholar]
- Hatini V, Tao W, Lai E. Expression of winged helix genes, BF-1 and BF-2, define adjacent domains within the developing forebrain and retina. J Neurobiol. 1994;25:1293–309. doi: 10.1002/neu.480251010. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev Biol. 1987;124:200–14. doi: 10.1016/0012-1606(87)90472-6. [DOI] [PubMed] [Google Scholar]
- Hicks D. Putative functions of fibroblast growth factors in retinal development, maturation and survival. Semin Cell Dev Biol. 1998;9:263–9. doi: 10.1006/scdb.1998.0230. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox- containing gene. Nature. 1991;354:522–5. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Hirst EM, Horsburgh G, Hetherington CM. Small eye (Sey): a mouse model for the genetic analysis of craniofacial abnormalities. Development. 1988;103(Suppl):115–9. doi: 10.1242/dev.103.Supplement.115. [DOI] [PubMed] [Google Scholar]
- Holash JA, Pasquale EB. Polarized expression of the receptor protein tyrosine kinase Cek5 in the developing avian visual system. Dev Biol. 1995;172:683–93. doi: 10.1006/dbio.1995.8039. [DOI] [PubMed] [Google Scholar]
- Holash JA, Soans C, Chong LD, Shao H, Dixit VM, Pasquale EB. Reciprocal expression of the Eph receptor Cek5 and its ligand(s) in the early retina. Dev Biol. 1997;182:256–69. doi: 10.1006/dbio.1996.8496. [DOI] [PubMed] [Google Scholar]
- Hollemann T, Bellefroid E, Pieler T. The Xenopus homologue of the Drosophila gene tailless has a function in early eye development. Development. 1998;125:2425–32. doi: 10.1242/dev.125.13.2425. [DOI] [PubMed] [Google Scholar]
- Horsford DJ, Nguyen MT, Sellar GC, Kothary R, Arnheiter H, McInnes RR. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development. 2005;132:177–87. doi: 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Gage FH. Epigenetic control of neural stem cell fate. Curr Opin Genet Dev. 2004;14:461–9. doi: 10.1016/j.gde.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–71. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Huillard E, Laugier D, Marx M. Defects in chicken neuroretina misexpressing the BMP antagonist Drm/Gremlin. Dev Biol. 2005 doi: 10.1016/j.ydbio.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Hyatt GA, Dowling JE. Retinoic acid. A key molecule for eye and photoreceptor development. Invest Ophthalmol Vis Sci. 1997;38:1471–5. [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Fadool JM, Dowling JE. Retinoic acid alters photoreceptor development in vivo. Proc Natl Acad Sci U S A. 1996a;93:13298–303. doi: 10.1073/pnas.93.23.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Marsh-Armstrong N, McCaffery P, Drager UC, Dowling JE. Retinoic acid establishes ventral retinal characteristics. Development. 1996b;122:195–204. doi: 10.1242/dev.122.1.195. [DOI] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Marsh-Armstrong NR, Dowling JE. Retinoic acid-induced duplication of the zebrafish retina. Proc Natl Acad Sci U S A. 1992;89:8293–7. doi: 10.1073/pnas.89.17.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer J. Looking at an oft-overlooked part of the eye: a new perspective on ciliary body development in chick. Dev Neurosci. 2004;26:456–65. doi: 10.1159/000082287. [DOI] [PubMed] [Google Scholar]
- Hyer J, Kuhlman J, Afif E, Mikawa T. Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Dev Biol. 2003;259:351–63. doi: 10.1016/s0012-1606(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–9. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Iwano H, Nakamura M, Tajima S. Xenopus MBD3 plays a crucial role in an early stage of development. Dev Biol. 2004;268:416–28. doi: 10.1016/j.ydbio.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Jean D, Ewan K, Gruss P. Molecular regulators involved in vertebrate eye development. Mech Dev. 1998;76:3–18. doi: 10.1016/s0925-4773(98)00117-8. [DOI] [PubMed] [Google Scholar]
- Jena N, Martin-Seisdedos C, McCue P, Croce CM. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res. 1997;230:28–37. doi: 10.1006/excr.1996.3411. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–9. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Burrus LW, Erickson CA. The expression patterns of Wnts and their antagonists during avian eye development. Mech Dev. 2002;116:173–6. doi: 10.1016/s0925-4773(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Jordan T, Hanson I, Zaletayev D, Hodgson S, Prosser J, Seawright A, Hastie N, van Heyningen V. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992;1:328–32. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- Kagiyama Y, Gotouda N, Sakagami K, Yasuda K, Mochii M, Araki M. Extraocular dorsal signal affects the developmental fate of the optic vesicle and patterns the optic neuroepithelium. Dev Growth Differ. 2005;47:523–36. doi: 10.1111/j.1440-169X.2005.00828.x. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Luo G, Hofmann C, Bradley A. BMP 7 is required for nephrogenesis, eye development, and skeletal patterning. Ann N Y Acad Sci. 1996;785:98–107. doi: 10.1111/j.1749-6632.1996.tb56247.x. [DOI] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kessler DS, Melton DA. Vertebrate embryonic induction: mesodermal and neural patterning. Science. 1994;266:596–604. doi: 10.1126/science.7939714. [DOI] [PubMed] [Google Scholar]
- Khosrowshahian F, Wolanski M, Chang WY, Fujiki K, Jacobs L, Crawford MJ. Lens and retina formation require expression of Pitx3 in Xenopus pre-lens ectoderm. Dev Dyn. 2005;234:577–89. doi: 10.1002/dvdy.20540. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lemke G. Hedgehog-regulated localization of Vax2 controls eye development. Genes Dev. 2006;20:2833–47. doi: 10.1101/gad.1462706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Koshiba-Takeuchi K, Takeuchi JK, Matsumoto K, Momose T, Uno K, Hoepker V, Ogura K, Takahashi N, Nakamura H, Yasuda K, Ogura T. Tbx5 and the retinotectum projection. Science. 2000;287:134–7. doi: 10.1126/science.287.5450.134. [DOI] [PubMed] [Google Scholar]
- Kurose H, Okamoto M, Shimizu M, Bito T, Marcelle C, Noji S, Ohuchi H. FGF19-FGFR4 signaling elaborates lens induction with the FGF8-L-Maf cascade in the chick embryo. Dev Growth Differ. 2005;47:213–23. doi: 10.1111/j.1440-169X.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–91. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Lapidot MPY. Genome-wide natural antisense transcription: coupling its regulation to its different regulatory mechanisms. EMBO Rep. 2006 Dec;7:1216–22. doi: 10.1038/sj.embor.7400857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leconte L, Lecoin L, Martin P, Saule S. Pax6 interacts with cVax and Tbx5 to establish the dorsoventral boundary of the developing eye. J Biol Chem. 2004;279:47272–7. doi: 10.1074/jbc.M406624200. [DOI] [PubMed] [Google Scholar]
- Lee HS, Bong YS, Moore KB, Soria K, Moody SA, Daar IO. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat Cell Biol. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- Lee HY, Wroblewski E, Philips GT, Stair CN, Conley K, Reedy M, Mastick GS, Brown NL. Multiple requirements for Hes 1 during early eye formation. Dev Biol. 2005;284:464–78. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Tierney C, Wen L, Wu JY, Rao Y. A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate. Development. 1997;124:603–15. doi: 10.1242/dev.124.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Yang JM, Jacobson RD, Pasko D, Sundin O. Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Dev Biol. 1994;162:181–94. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- Li X, Carthew RW. A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell. 2005;123:1267–77. doi: 10.1016/j.cell.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Lin SY, Johnson SM, Abraham M, Vella MC, Pasquinelli A, Gamberi C, Gottlieb E, Slack FJ. The C elegans hunchback homolog, hbl-1, controls temporal patterning and is a probable microRNA target. Dev Cell. 2003;4:639–50. doi: 10.1016/s1534-5807(03)00124-2. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–34. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Hunt SP. Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol Cell Neurosci. 1997;8:417–29. doi: 10.1006/mcne.1997.0598. [DOI] [PubMed] [Google Scholar]
- Loosli F, Staub W, Finger-Baier KC, Ober EA, Verkade H, Wittbrodt J, Baier H. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003;4:894–9. doi: 10.1038/sj.embor.embor919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F, Winkler S, Burgtorf C, Wurmbach E, Ansorge W, Henrich T, Grabher C, Arendt D, Carl M, Krone A, Grzebisz E, Wittbrodt J. Medaka eyeless is the key factor linking retinal determination and eye growth. Development. 2001;128:4035–44. doi: 10.1242/dev.128.20.4035. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–20. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Luo T, Sakai Y, Wagner E, Drager UC. Retinoids, eye development, and maturation of visual function. J Neurobiol. 2006;66:677–86. doi: 10.1002/neu.20239. [DOI] [PubMed] [Google Scholar]
- Lupo G, Andreazzoli M, Gestri G, Liu Y, He RQ, Barsacchi G. Homeobox genes in the genetic control of eye development. Int J Dev Biol. 2000;44:627–36. [PubMed] [Google Scholar]
- Lupo G, Harris WA, Lewis KE. Mechanisms of ventral patterning in the vertebrate nervous system. Nat Rev Neurosci. 2006;7:103–14. doi: 10.1038/nrn1843. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–78. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Marcus RC, Gale NW, Morrison ME, Mason CA, Yancopoulos GD. Eph family receptors and their ligands distribute in opposing gradients in the developing mouse retina. Dev Biol. 1996;180:786–9. doi: 10.1006/dbio.1996.0347. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, McCaffery P, Gilbert W, Dowling JE, Drager UC. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc Natl Acad Sci U S A. 1994;91:7286–90. doi: 10.1073/pnas.91.15.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Rodrigo I, Bovolenta P. Eye development: a view from the retina pigmented epithelium. Bioessays. 2004;26:766–77. doi: 10.1002/bies.20064. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Jamrich M. Regulation of eye formation by the Rx and Pax6 homeobox genes. Cell Mol Life Sci. 2000;57:186–94. doi: 10.1007/PL00000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Osumi-Yamashita N, Noji S, Ohuchi H, Koyama E, Myokai F, Matsuo N, Taniguchi S, Doi H, Iseki S, et al. A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain crest cells. Nat Genet. 1993;3:299–304. doi: 10.1038/ng0493-299. [DOI] [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Wagner E, O’Neil J, Petkovich M, Drager UC. Dorsal and ventral rentinoic territories defined by retinoic acid synthesis, break-down and nuclear receptor expression. Mech Dev. 1999;85:203–14. doi: 10.1016/s0925-4773(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Menko S, Philp N, Veneziale B, Walker J. Integrins and development: how might these receptors regulate differentiation of the lens. Ann N Y Acad Sci. 1998;842:36–41. doi: 10.1111/j.1749-6632.1998.tb09629.x. [DOI] [PubMed] [Google Scholar]
- Mey J, McCaffery P, Klemeit M. Sources and sink of retinoic acid in the embryonic chick retina: distribution of aldehyde dehydrogenase activities, CRABP-I, and sites of retinoic acid inactivation. Brain Res Dev Brain Res. 2001;127:135–48. doi: 10.1016/s0165-3806(01)00127-4. [DOI] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Molotkova N, Duester G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev Dyn. 2004;231:270–7. doi: 10.1002/dvdy.20128. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–10. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SA. To differentiate or not to differentiate: regulation of cell fate decisions by being in the right place at the right time. Cell Cycle. 2004;3:564–6. [PubMed] [Google Scholar]
- Moore KB, Mood K, Daar IO, Moody SA. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev Cell. 2004;6:55–67. doi: 10.1016/s1534-5807(03)00395-2. [DOI] [PubMed] [Google Scholar]
- Moore KB, Schneider ML, Vetter ML. Posttranslational mechanisms control the timing of bHLH function and regulate retinal cell fate. Neuron. 2002;34:183–95. doi: 10.1016/s0896-6273(02)00666-9. [DOI] [PubMed] [Google Scholar]
- Morcillo J, Martinez-Morales JR, Trousse F, Fermin Y, Sowden JC, Bovolenta P. Proper patterning of the optic fissure requires the sequential activity of BMP7 and SHH. Development. 2006;133:3179–90. doi: 10.1242/dev.02493. [DOI] [PubMed] [Google Scholar]
- Mui SH, Kim JW, Lemke G, Bertuzzi S. Vax genes ventralize the embryonic eye. Genes Dev. 2005;19:1249–59. doi: 10.1101/gad.1276605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Albert S, Blader P, Fischer N, Hallonet M, Strahle U. Direct action of the nodal-related signal cyclops in induction of sonic hedgehog in the ventral midline of the CNS. Development. 2000;127:3889–97. doi: 10.1242/dev.127.18.3889. [DOI] [PubMed] [Google Scholar]
- Ogino H, Yasuda K. Sequential activation of transcription factors in lens induction. Dev Growth Differ. 2000;42:437–48. doi: 10.1046/j.1440-169x.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- Ogita J, Isogai E, Sudo H, Sakiyama S, Nakagawara A, Koseki H. Expression of the Dan gene during chicken embryonic development. Mech Dev. 2001;109:363–5. doi: 10.1016/s0925-4773(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Ohsaki K, Morimitsu T, Ishida Y, Kominami R, Takahashi N. Expression of the Vax family homeobox genes suggests multiple roles in eye development. Genes Cells. 1999;4:267–76. doi: 10.1046/j.1365-2443.1999.00257.x. [DOI] [PubMed] [Google Scholar]
- Onwochei BC, Simon JW, Bateman JB, Couture KC, Mir E. Ocular colobomata. Surv Ophthalmol. 2000;45:175–94. doi: 10.1016/s0039-6257(00)00151-x. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Kessel M. Patterning of the chick forebrain anlage by the prechordal plate. Development. 1997;124:4153–62. doi: 10.1242/dev.124.20.4153. [DOI] [PubMed] [Google Scholar]
- Peters MA. Patterning the neural retina. Curr Opin Neurobiol. 2002;12:43–8. doi: 10.1016/s0959-4388(02)00288-x. [DOI] [PubMed] [Google Scholar]
- Peters MA, Cepko CL. The dorsal-ventral axis of the neural retina is divided into multiple domains of restricted gene expression which exhibit features of lineage compartments. Dev Biol. 2002;251:59–73. doi: 10.1006/dbio.2002.0791. [DOI] [PubMed] [Google Scholar]
- Petrausch B, Jung M, Leppert CA, Stuermer CA. Lesion-induced regulation of netrin receptors and modification of netrin-1 expression in the retina of fish and grafted rats. Mol Cell Neurosci. 2000;16:350–64. doi: 10.1006/mcne.2000.0877. [DOI] [PubMed] [Google Scholar]
- Porter FD, Drago J, Xu Y, Cheema SS, Wassif C, Huang SP, Lee E, Grinberg A, Massalas JS, Bodine D, Alt F, Westphal H. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development. 1997;124:2935–44. doi: 10.1242/dev.124.15.2935. [DOI] [PubMed] [Google Scholar]
- Quinn JC, West JD, Hill RE. Multiple functions for Pax6 in mouse eye and nasal development. Genes Dev. 1996;10:435–46. doi: 10.1101/gad.10.4.435. [DOI] [PubMed] [Google Scholar]
- Rembold M, Loosli F, Adams RJ, Wittbrodt J. Individual cell migration serves as the driving force for optic vesicle evagination. Science. 2006;313:1130–4. doi: 10.1126/science.1127144. [DOI] [PubMed] [Google Scholar]
- Reza HM, Yasuda K. The involvement of neural retina pax6 in lens fiber differentiation. Dev Neurosci. 2004;26:318–27. doi: 10.1159/000082273. [DOI] [PubMed] [Google Scholar]
- Ryan DG, Oliveira-Fernandes M, Lavker RM. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–84. [PubMed] [Google Scholar]
- Saha MS, Spann CL, Grainger RM. Embryonic lens induction: more than meets the optic vesicle. Cell Differ Dev. 1989;28:153–71. doi: 10.1016/0922-3371(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura S, Yamamoto TS, Ueno N, Noda M. Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science. 2001;293:111–5. doi: 10.1126/science.1058379. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Takahashi H, Shintani T, Etani K, Aoshima A, Noda M. Role of bone morphogenic protein 2 in retinal patterning and retinotectal projection. J Neurosci. 2006;26:10868–78. doi: 10.1523/JNEUROSCI.3027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa S, Takabatake T, Takabatake Y, Muramatsu T, Takeshima K. Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. Genesis. 2002;33:86–96. doi: 10.1002/gene.10095. [DOI] [PubMed] [Google Scholar]
- Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie ND. Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996;86:71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Schook P. Morphogenetic movements during the early development of the chick eye. An ultrastructural and spatial reconstructive study. B. Invagination of the optic vesicle and fusion of its walls. Acta Morphol Neerl Scand. 1980;18:159–80. [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–9. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Schulte D, Furukawa T, Peters MA, Kozak CA, Cepko CL. Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron. 1999;24:541–53. doi: 10.1016/s0896-6273(00)81111-3. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–34. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Sen J, Harpavat S, Peters MA, Cepko CL. Retinoic acid regulates the expression of dorsoventral topographic guidance molecules in the chick retina. Development. 2005;132:5147–59. doi: 10.1242/dev.02100. [DOI] [PubMed] [Google Scholar]
- Skowronska-Krawczyk D, Ballivet M, Dynlacht BD, Matter JM. Highly specific interactions between bHLH transcription factors and chromatin during retina development. Development. 2004;131:4447–4454. doi: 10.1242/dev.01302. [DOI] [PubMed] [Google Scholar]
- Solursh M, Langille RM, Wood J, Sampath TK. Osteogenic protein-1 is required for mammalian eye development. Biochem Biophys Res Commun. 1996;218:438–43. doi: 10.1006/bbrc.1996.0078. [DOI] [PubMed] [Google Scholar]
- Song L, Tuan RS. MicroRNAs and cell differentiation in mammalian development. Birth Defects Res C Embryo Today. 2006;78:140–9. doi: 10.1002/bdrc.20070. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev. 1999;85:85–96. doi: 10.1016/s0925-4773(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Stull DL, Wikler KC. Retinoid-dependent gene expression regulates early morphological events in the development of the murine retina. J Comp Neurol. 2000;417:289–98. doi: 10.1002/(sici)1096-9861(20000214)417:3<289::aid-cne3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Taniguchi M, Yagi T, Akagi Y, Nojyo Y, Tamamaki N. Guidance of glial precursor cell migration by secreted cues in the developing optic nerve. Development. 2001;128:3321–30. doi: 10.1242/dev.128.17.3321. [DOI] [PubMed] [Google Scholar]
- Sullivan CH, Braunstein L, Hazard-Leonards RM, Holen AL, Samaha F, Stephens L, Grainger RM. A re-examination of lens induction in chicken embryos: in vitro studies of early tissue interactions. Int J Dev Biol. 2004;48:771–82. doi: 10.1387/ijdb.041894cs. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Shintani T, Sakuta H, Kato A, Ohkawara T, Osumi N, Noda M. Identification of RALDH-3, a novel retinaldehyde dehydrogenase, expressed in the ventral region of the retina. Mech Dev. 2000;98:37–50. doi: 10.1016/s0925-4773(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Svoboda KK, O’Shea KS. Optic vesicle defects induced by vincristine sulfate: an in vivo and in vitro study in the mouse embryo. Teratology. 1984;29:223–39. doi: 10.1002/tera.1420290209. [DOI] [PubMed] [Google Scholar]
- Svoboda KK, O’Shea KS. An analysis of cell shape and the neuroepithelial basal lamina during optic vesicle formation in the mouse embryo. Development. 1987;100:185–200. doi: 10.1242/dev.100.2.185. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Shintani T, Sakuta H, Noda M. CBF1 controls the retinotectal topographical map along the anteroposterior axis through multiple mechanisms. Development. 2003;130:5203–15. doi: 10.1242/dev.00724. [DOI] [PubMed] [Google Scholar]
- Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–68. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Thanos S, Mey J. Development of the visual system of the chick. II. Mechanisms of axonal guidance. Brain Res Brain Res Rev. 2001;35:205–45. doi: 10.1016/s0165-0173(01)00049-2. [DOI] [PubMed] [Google Scholar]
- Tomita K, Ishibashi M, Nakahara K, Ang SL, Nakanishi S, Guillemot F, Kageyama R. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron. 1996;16:723–34. doi: 10.1016/s0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–91. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Trousse F, Esteve P, Bovolenta P. Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci. 2001;21:1292–301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci. 2004;26:352–8. doi: 10.1159/000082277. [DOI] [PubMed] [Google Scholar]
- Varga ZM, Wegner J, Westerfield M. Anterior movement of ventral diencephalic precursors separates the primordial eye field in the neural plate and requires cyclops. Development. 1999;126:5533–46. doi: 10.1242/dev.126.24.5533. [DOI] [PubMed] [Google Scholar]
- Viczian AS, Bang AG, Harris WA, Zuber ME. Expression of Xenopus laevis Lhx2 during eye development and evidence for divergent expression among vertebrates. Dev Dyn. 2006;235:1133–41. doi: 10.1002/dvdy.20708. [DOI] [PubMed] [Google Scholar]
- Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A. 2005;102:16426–31. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Hopker A, Momose T, Rohrer H, Yasuda K, Ishihara L, Rapaport DH. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech Dev. 2000;94:25–36. doi: 10.1016/s0925-4773(00)00320-8. [DOI] [PubMed] [Google Scholar]
- Wagner E, McCaffery P, Drager UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev Biol. 2000;222:460–70. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–49. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–88. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Werner Natural antisense transcripts. RNA Biol. 2005 doi: 10.4161/rna.2.2.1852. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–81. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–35. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–8. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa J, Hirano S, Yamagata M, Noda M. Visual projection map specified by topographic expression of transcription factors in the retina. Nature. 1996;382:632–5. doi: 10.1038/382632a0. [DOI] [PubMed] [Google Scholar]
- Zaghloul NA, Yan B, Moody SA. Step-wise specification of retinal stem cells during normal embryogenesis. Biol Cell. 2005;97:321–37. doi: 10.1042/BC20040521. [DOI] [PubMed] [Google Scholar]
- Zelenka PS. Regulation of cell adhesion and migration in lens development. Int J Dev Biol. 2004;48:857–65. doi: 10.1387/ijdb.041871pz. [DOI] [PubMed] [Google Scholar]
- Zhang L, Mathers PH, Jamrich M. Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis. 2000;28:135–42. [PubMed] [Google Scholar]
- Zhang SS, Fu XY, Barnstable CJ. Molecular aspects of vertebrate retinal development. Mol Neurobiol. 2002;26:137–52. doi: 10.1385/MN:26:2-3:137. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001;233:271–90. doi: 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Hung FC, Colvin JS, White A, Dai W, Lovicu FJ, Ornitz DM, Overbeek PA. Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development. 2001;128:5051–60. doi: 10.1242/dev.128.24.5051. [DOI] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–67. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]


