Abstract
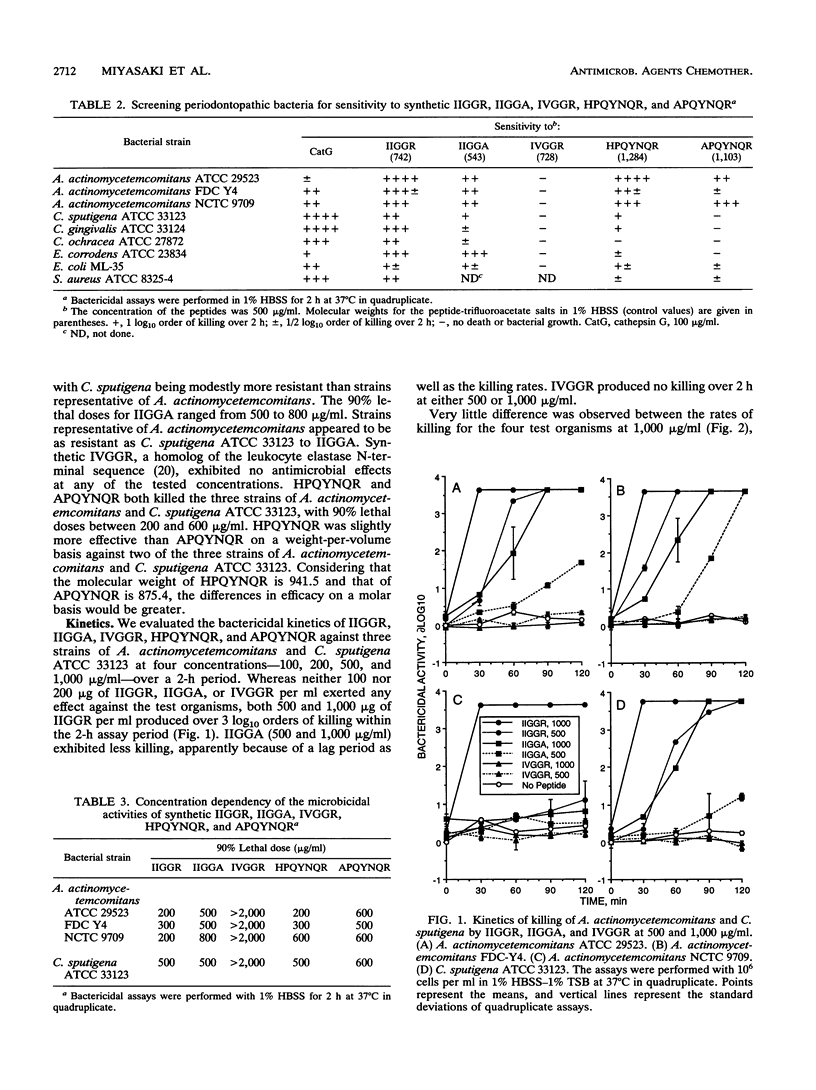
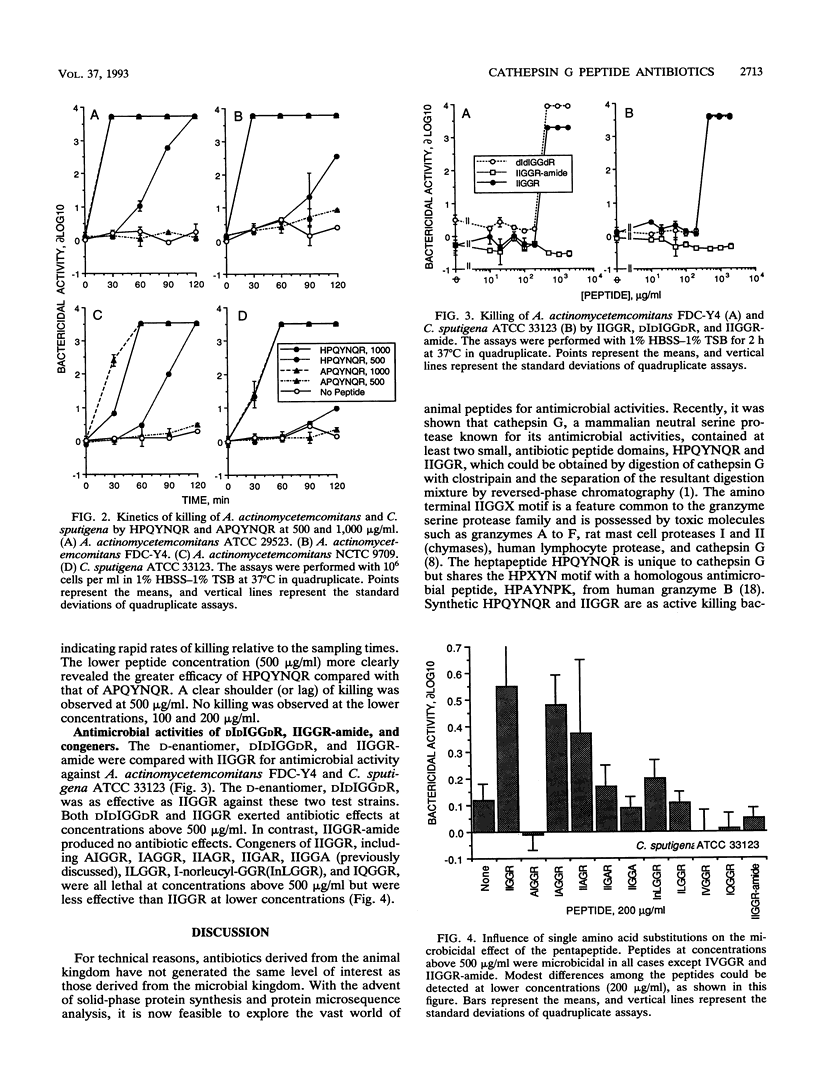
Actinobacillus actinomycetemcomitans and Capnocytophaga spp. are gram-negative bacteria implicated in the etiology of periodontal disease (particularly in individuals with neutrophil defects) and life-threatening systemic infections. They are resistant to many antibiotics of microbial origin but are sensitive to the nonoxidative microbicidal action of neutrophils. These organisms are susceptible to the microbicidal effect of cathepsin G but are killed by two distinct mechanisms. The purpose of this study was to assess their sensitivity to the antibiotic effects of IIGGR and HPQYNQR, antimicrobial peptides derived from human neutrophil cathepsin G. The efficacies of the synthetic peptides IIGGR and HPQYNQR were tested by single-dose screening, dose-response, and kinetic assays against three representative strains (each) of A. actinomycetemcomitans and Capnocytophaga spp. and one strain of Eikenella corrodens. Strains of A. actinomycetemcomitans were sensitive to IIGGR and HPQYNQR at equal concentrations (wt/vol), whereas strains of Capnocytophaga and E. corrodens were more sensitive to IIGGR than to HPQYNQR. These differential antibiotic effects occurred over both time and dose ranges too narrow to be of therapeutic significance but are consistent with the premise that cathepsin G kills these oral bacteria by two distinct mechanisms. Except for IVGGR, congeners of IIGGR, including AIGGR, IAGGR, IIAGR, IIGAR, IIGGA, IQGGR, ILGGR, and I-norleucyl-GGR (InLGGR), were microbicidal at 500 micrograms/ml. IIGGR-amide exhibited no antibiotic activity. The D-enantiomer of IIGGR, DIDIGGDR, was as potent as IIGGR itself. APQYNQR exhibited antibiotic activity but somewhat less than HPQYNQR. We conclude that charge distribution, but not chirality or net charge, is an important determinant in the antibiotic efficacy of IIGGR. Moreover, peptide antibiotics derived from cathepsin G may have therapeutic value against periodontal gram-negative, facultative bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangalore N., Travis J., Onunka V. C., Pohl J., Shafer W. M. Identification of the primary antimicrobial domains in human neutrophil cathepsin G. J Biol Chem. 1990 Aug 15;265(23):13584–13588. [PubMed] [Google Scholar]
- Boman H. G. Antibacterial peptides: key components needed in immunity. Cell. 1991 Apr 19;65(2):205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- Boomer S., Coulter W. A., Guthrie D. J. Effects on oral bacteria of a peptide derived from human cathepsin G. Biochem Soc Trans. 1992 Feb;20(1):61S–61S. doi: 10.1042/bst020061s. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Smith S. M., Goldstein E. J., Miyasaki K. T., Quah S. E., Buccini F. Failure of vancomycin prophylaxis and treatment for Actinobacillus actinomycetemcomitans endocarditis. Antimicrob Agents Chemother. 1986 Apr;29(4):699–700. doi: 10.1128/aac.29.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgaard H., Ostergaard E., Bayne S., Svendsen A., Thomsen J., Engels M., Wollmer A. Covalent structure of two novel neutrophile leucocyte-derived proteins of porcine and human origin. Neutrophile elastase homologues with strong monocyte and fibroblast chemotactic activities. Eur J Biochem. 1991 Apr 23;197(2):535–547. doi: 10.1111/j.1432-1033.1991.tb15942.x. [DOI] [PubMed] [Google Scholar]
- Forlenza S. W., Newman M. G., Lipsey A. I., Siegel S. E., Blachman U. Capnocytophaga sepsis: a newly recognised clinical entity in granulocytopenic patients. Lancet. 1980 Mar 15;1(8168 Pt 1):567–568. doi: 10.1016/s0140-6736(80)91057-0. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988 Mar;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Kalmar J. R., Arnold R. R., van Dyke T. E. Direct interaction of Actinobacillus actinomycetemcomitans with normal and defective (LJP) neutrophils. J Periodontal Res. 1987 May;22(3):179–181. doi: 10.1111/j.1600-0765.1987.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Kaplan A. H., Weber D. J., Oddone E. Z., Perfect J. R. Infection due to Actinobacillus actinomycetemcomitans: 15 cases and review. Rev Infect Dis. 1989 Jan-Feb;11(1):46–63. doi: 10.1093/clinids/11.1.46. [DOI] [PubMed] [Google Scholar]
- Miyasaki K. T., Bodeau A. L., Ganz T., Selsted M. E., Lehrer R. I. In vitro sensitivity of oral, gram-negative, facultative bacteria to the bactericidal activity of human neutrophil defensins. Infect Immun. 1990 Dec;58(12):3934–3940. doi: 10.1128/iai.58.12.3934-3940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki K. T., Bodeau A. L. In vitro killing of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. by human neutrophil cathepsin G and elastase. Infect Immun. 1991 Sep;59(9):3015–3020. doi: 10.1128/iai.59.9.3015-3020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti D. M., Snydman D. R. Capnocytophaga species: infections in nonimmunocompromised and immunocompromised hosts. J Infect Dis. 1985 Jan;151(1):140–147. doi: 10.1093/infdis/151.1.140. [DOI] [PubMed] [Google Scholar]
- Pereira H. A., Erdem I., Pohl J., Spitznagel J. K. Synthetic bactericidal peptide based on CAP37: a 37-kDa human neutrophil granule-associated cationic antimicrobial protein chemotactic for monocytes. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4733–4737. doi: 10.1073/pnas.90.10.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. A., Shafer W. M., Pohl J., Martin L. E., Spitznagel J. K. CAP37, a human neutrophil-derived chemotactic factor with monocyte specific activity. J Clin Invest. 1990 May;85(5):1468–1476. doi: 10.1172/JCI114593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V. C. Mechanism of staphylococcal resistance to non-oxidative antimicrobial action of neutrophils: importance of pH and ionic strength in determining the bactericidal action of cathepsin G. J Gen Microbiol. 1989 Apr;135(4):825–830. doi: 10.1099/00221287-135-4-825. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Pohl J., Onunka V. C., Bangalore N., Travis J. Human lysosomal cathepsin G and granzyme B share a functionally conserved broad spectrum antibacterial peptide. J Biol Chem. 1991 Jan 5;266(1):112–116. [PubMed] [Google Scholar]
- Shafer W. M., Shepherd M. E., Boltin B., Wells L., Pohl J. Synthetic peptides of human lysosomal cathepsin G with potent antipseudomonal activity. Infect Immun. 1993 May;61(5):1900–1908. doi: 10.1128/iai.61.5.1900-1908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Watorek W., Karr S., Giles J., Bode W., Travis J. Primary structure of human neutrophil elastase. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2228–2232. doi: 10.1073/pnas.84.8.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Salient Biochemical Characters of Actinobacillus actinomycetemcomitans. Arch Microbiol. 1982 Feb;131(1):60–67. doi: 10.1007/BF00451500. [DOI] [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiluk K. R., Skubitz K. M., Gray B. H. Comparison of granule proteins from human polymorphonuclear leukocytes which are bactericidal toward Pseudomonas aeruginosa. Infect Immun. 1991 Nov;59(11):4193–4200. doi: 10.1128/iai.59.11.4193-4200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. G., Snable J. L., Griffith J. E., Scott R. W. Characterization of two azurphil granule proteases with active-site homology to neutrophil elastase. J Biol Chem. 1990 Feb 5;265(4):2038–2041. [PubMed] [Google Scholar]
- Zambon J. J., Umemoto T., De Nardin E., Nakazawa F., Christersson L. A., Genco R. J. Actinobacillus actinomycetemcomitans in the pathogenesis of human periodontal disease. Adv Dent Res. 1988 Nov;2(2):269–274. doi: 10.1177/08959374880020021101. [DOI] [PubMed] [Google Scholar]


