Abstract
Daidzin, a major active principle of an ancient Chinese herbal treatment (Radix puerariae) for alcohol abuse, selectively suppresses ethanol intake in all rodent models tested. It also inhibits mitochondrial aldehyde dehydrogenase (ALDH-2). Studies on ethanol intake suppression and ALDH-2 inhibition by structural analogs of daidzin established a link between these two activities and suggested that daidzin may suppress ethanol intake by inhibiting ALDH-2. ALDH-2 is a principal enzyme involved in serotonin (5-HT) and dopamine (DA) metabolism. Thus, daidzin may act by inhibiting 5-HT and DA metabolism. To evaluate this possibility, we have studied the effect of daidzin and its analogs on 5-HT and DA metabolism in isolated hamster and rat liver mitochondria. Daidzin potently inhibits the formation of 5-hydroxyindole-3-acetic acid (5-HIAA) and 3,4-dihydroxyphenylacetic acid (DOPAC) from their respective amines in isolated mitochondria. Inhibition is concentration-dependent and is accompanied by a concomitant accumulation of 5-hydroxyindole-3-acetaldehyde and 3,4-dihydroxyphenylacetaldehyde. Daidzin analogs that suppress hamster ethanol intake also inhibit 5-HIAA and DOPAC formation. Comparing their effects on mitochondria-catalyzed 5-HIAA or DOPAC formation and hamster ethanol intake reveals a positive correlation—the stronger the inhibition on 5-HIAA or DOPAC formation, the greater the ethanol intake suppression. Daidzin and its active analogs, at concentrations that significantly inhibit 5-HIAA formation, have little or no effect on mitochondria-catalyzed 5-HT depletion. It appears that the antidipsotropic action of daidzin is not mediated by 5-HT (or DA) but rather by its reactive intermediates 5-hydroxyindole-3-acetaldehyde and, presumably, 3,4-dihydroxyphenylacetaldehyde as well, which accumulates in the presence of daidzin.
In a recent study, we demonstrated that daidzin, a constituent of an ancient Chinese herbal treatment (Radix puerariae) for alcohol abuse, selectively suppresses home-cage ethanol intake by Syrian golden hamsters under a two-bottle, free choice (ethanol/water) condition (1). Since then, the ethanol intake suppressive (antidipsotropic) activity of daidzin and the R. puerariae extract have been confirmed by us (2, 3) and other investigators (4–6) independently in golden hamsters, Wistar rats, Fawn hooded rats, and the genetically bred P rats under various experimental conditions, including two-lever choice (ethanol/starch solution), two-bottle free choice (ethanol/water), limited access, and ethanol-deprived paradigms. These laboratory findings are consistent with those of the herbal treatment used in China and thereby suggest that daidzin or one or more of its derivatives and/or metabolites might be effective in the treatment of alcohol abuse and/or alcoholism.
The mechanism by which daidzin selectively suppresses ethanol intake in laboratory animals is unknown at this time. In an early study, we showed that daidzin is a selective and potent inhibitor of mitochondrial aldehyde dehydrogenase (ALDH-2) (7). ALDH-2 catalyzes the detoxification of acetaldehyde, an intermediate of ethanol metabolism (8). Some humans inherit an inactive variant form of ALDH-2, and in these individuals alcohol abuse is rare (9–11). Based on these findings, we postulated that daidzin may act by mimicking the consequences of the apparently harmless natural mutation of the ALDH-2 gene (1). To evaluate this hypothesis, we have synthesized a series of structural analogs of daidzin and tested and compared their ALDH-2 inhibitory activity with their antidipsotropic activity. Early results demonstrated a direct correlation between the two and raised the possibility that daidzin may, in fact, suppress ethanol intake by inhibiting ALDH-2 (12).
By inhibiting ALDH-2, daidzin could in principle suppress ethanol consumption by at least two routes. On the one hand, it might act as an ethanol-sensitizing agent that discourages ethanol consumption by inhibiting acetaldehyde metabolism subsequent to drinking and thereby allow it to reach toxic levels. On the other hand, it could perturb an as-yet-undefined physiological pathway catalyzed by ALDH-2 and alter the concentrations of some endogenous substrate(s) that regulate ethanol drinking behavior. To determine whether or not daidzin suppresses hamster ethanol consumption by inhibiting acetaldehyde metabolism, we studied the effect of daidzin on acetaldehyde clearance in hamsters challenged with ethanol. This study showed that daidzin, at a dose that significantly suppresses ethanol consumption, does not affect overall acetaldehyde metabolism (13), and we ruled out the ethanol-sensitizing mechanism for daidzin.
It has long been postulated that ALDH-2 is involved in the oxidation of aldehydes that derive from biologically active monoamines such as serotonin (5-HT) and dopamine (DA) in mammalian brain tissue via the action of monoamine oxidase (MAO) (14, 15). Studies on DA metabolism in isolated mitochondria and various subcellular fractions identified ALDH-2 as the principal enzyme that catalyzes the oxidation of DA-derived 3,4-dihydroxyphenylacetaldehyde (DOPAL) in rat liver (16). Recent kinetic analyses have shown that both DOPAL and 5-hydroxyindole-3-acetaldehyde (5-HIAL) are excellent substrates for ALDH-2 (17). This further reinforces the belief that ALDH-2 is directly involved in the metabolism of monoamine neurotransmitters. To elucidate the mechanism of action of daidzin, we have studied the effect of daidzin and its structural analogs on 5-HT and DA metabolism by using isolated hamster liver mitochondria. Rats and golden hamsters respond differently to puerarin (8-C-glucosyl-daidzein), another isoflavone found in R. puerariae. Puerarin appears to suppress ethanol intake in rats (4) but has little or no effect on that in golden hamsters (1). Therefore, the effects of puerarin and other daidzin analogs on 5-HT and DA metabolism in isolated rat liver mitochondria also were examined for comparison.
MATERIALS AND METHODS
Mature male Syrian golden hamsters (100–130 g) and Wistar rats (200–250 g) were obtained from Harlan—Sprague–Dawley. Animals were housed (three or four per cage) in a room maintained at 23°C on a 12/12 light/dark cycle with ad libitum access to tap water and Purina Rodent Laboratory Chow 5001. Animals were killed in a CO2 chamber, and livers were removed and used immediately for the preparation of isolated mitochondria. Daidzin, daidzein, and their synthetic analogs were prepared as described (12, 18, 19) and were identified by mass and NMR spectroscopy. Puerarin was purchased from Indofine Chemical (Somerville, NJ). 5-HT, 5-hydroxytryptophol (5-HTOL), 5-HIAA, DA, and DOPAC were products of Research Biochemicals (Natick, MA). DOPAL and 5-HIAL were prepared according to the procedure of Nilsson and Tottmar (20). 3,4-dihydroxyphenylethanol (DOPET) was prepared by reduction of DOPAL by using sodium borohydride. All other reagents used were of the best grade available.
5-HT and DA Metabolism in Isolated Liver Mitochondria.
Mitochondrial preparations were obtained by the method of Johnson and Lardy (21). The integrity of these mitochondrial preparations, evaluated by measuring their latent glutamate dehydrogenase activity (22) before and after the metabolic study, was over 97% and 93%, respectively. 5-HT and DA metabolisms in the mitochondrial preparations were assayed by monitoring the formation of 5-HIAA and DOPAC, respectively, and/or the depletion of their respective amines in a 0.5-ml standard assay medium containing 10 mM Tris⋅HCl (pH 7.4), 0.3 M mannitol, 2.5 mM MgCl2, 10 mM K2HPO4, 10 mM KCl, and specified amounts of substrates, test compounds, and freshly prepared mitochondrial preparations. Reactions were initiated by the addition of mitochondria and allowed to proceed in a 37°C shaking water bath for 30 min. Reactions were terminated by the addition of 0.05 ml each of ice-cold 1 M HClO4 and 10 mM EDTA. The samples were kept on ice for 1 h followed by centrifugation (12,000 rpm) in a microcentrifuge (Microspin 24S, Sorvall) for 15 min. 5-HT, 5-HIAA, 5-HTOL, DA, DOPAC, and DOPET in the supernatant were analyzed directly by HPLC. 5-HIAL and DOPAL were converted to their semicarbazones before analysis.
HPLC Analysis.
The HPLC system used in this study consisted of a BAS (West Lafayette, IN) Sample Sentinel Autosampler with refrigerated (4°C) sample compartment, PM80 solvent delivery system, and a LC-26 on-line degasser. The detector is an LC-4C amperometric controller with a CC-5 cross-flow thin layer (0.005“) electrochemical cell comprised of glassy carbon and silver/silver chloride reference electrodes (Bioanlytical Systems, West Lafayette, IN). For routine analysis, the potential and sensitivity were set at 650 mV and 10 or 100 nA full scale, respectively. Column temperature was maintained with a Waters Temperature Control Module. DA, 5-HT, DOPAL–, and 5-HIAL–semicarbazone were analyzed on a BAS phase II ODS-3, 3-μm, 3.2 × 100-mm column. The column was developed at 30°C at 1 ml/min in a mobile phase that contained 1.23% monochloroacetic acid (Sigma), 0.02% sodium 1-octyl sulfate (Lancaster Synthesis), 0.025% EDTA–Na2, and 5% acetonitrile (vol/vol) (pH 3). The retention times for DOPAL–semicarbazone, 5-HIAL–semicarbazone, DA, and 5-HT were 4.6, 7.5, 10.4, and 17.6 min, respectively. DOPAC, DOPET, 5-HIAA, and 5-HTOL were analyzed on a Beckman Ultrasphere ODS-5, 5-μ, 4.6 × 250-mm column. The column was developed at 26°C at 1 ml/min in a mobile phase containing 1.23% monochloroacetic acid, 3% methanol (vol/vol), and 1% acetonitrile (vol/vol) (pH 2.1). The retention times for DOPET, DOPAC, 5-HTOL, and 5-HIAA were 6.3, 10.5, 16, and 19.3 min, respectively. Data were collected and analyzed with a Nelsen data collection system (Perkin–Elmer) or a Waters 740 Data Module.
Enzyme Assays.
The mitochondrial pellet obtained from 5 g of liver was resuspended in 10 ml of 10 mM sodium phosphate buffer (pH 7.4), kept on ice, and sonicated for 30 s at 90 W of power with a Branson Sonifier cell disruptor. This suspension was used for MAO, ALDH, alcohol dehydrogenase (ADH), and aldehyde reductase (AR) activity measurements. ADH and ALDH activities were assayed in 50 mM sodium phosphate buffer (pH 7.5) containing 2.4 mM NAD+, using 40 mM ethanol and 5 μM acetaldehyde as substrate, respectively. Activity was determined by following the increase in absorbance at 340 nm with a Varian Cary 1 spectrophotometer thermostated at 25°C (23). AR activity was assayed by following the decrease in absorbance at 340 nm in an assay mixture containing 0.2 mM NADPH and 0.2 mM p-nitrobenzaldehyde. One unit of activity is defined as the amount of enzyme that catalyzes the oxidation of 1 μmol of substrate per minute. Mitochondrial membranes, prepared as described by Nilsson and Tottmar (20), were used as a crude preparation of MAO. MAO activity was assayed by the same method described for the 5-HT and DA metabolism assay except that 5 mM semicarbazide was included in the incubation medium as an aldehyde trap.
RESULTS
MAO, ALDH-2, ADH, and AR Activity of Hamster and Rat Liver Mitochondrial Preparations.
Lysates of the hamster and rat liver mitochondrial preparations used in this study contained no detectable activity of ADH and AR but exhibited activity of both MAO and the low Km ADLH-2, the two principal enzymes thought to be involved in 5-HT and DA metabolism in vivo (16, 17). ALDH-2 activity of the hamster and rat liver preparations measured with 5 μM of acetaldehyde were 17 and 5.2 mU/mg of protein, respectively. MAO activities of hamster liver mitochondrial lysates measured in a standard assay medium containing 1 mM 5-HT or DA were 3 or 13.6 mU/mg protein, respectively, whereas those of the rat mitochondrial lysates were 8.4 or 25.2 mU/mg protein, determined with the two respective substrates.
5-HT Metabolism Catalyzed by Isolated Hamster and Rat Liver Mitochondria.
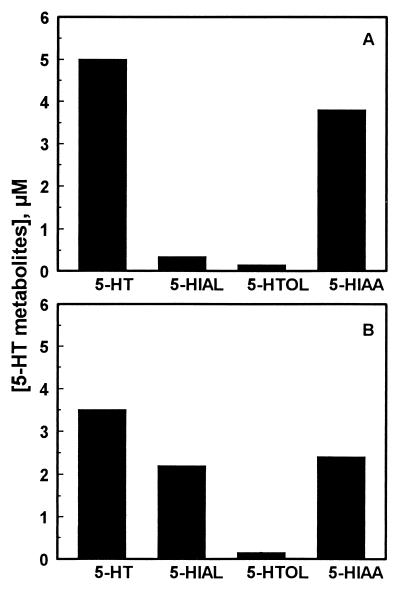
Hamster and rat liver mitochondrial preparations contained no detectable amounts of endogenous 5-HT, DA, or any of their known metabolites. When supplied with exogenous 5-HT, these preparations effectively metabolized this monoamine to its major metabolic product 5-HIAA. At a concentration of 0.4 mg/ml, hamster liver mitochondrial preparations metabolized 50% of the total 5-HT (10 μM) added in 30 min (Fig. 1A), and of that, 86% was recovered as the known metabolic intermediates and products of 5-HT, with 5-HIAA being most abundant (3.8 μM), followed by 5-HIAL (0.35 μM) and 5-HTOL (0.15 μM). Approximately 14% of the total 5-HT metabolized cannot be accounted for by known metabolic products and presumably is lost owing to the formation of adducts with mitochondrial proteins, membranes, and/or other condensation products via the reactive aldehyde intermediate 5-HIAL (20).
Figure 1.
5-HT metabolism in hamster (A) and rat (B) liver mitochondrial preparations. 5-HT (10 μM) was incubated with freshly prepared hamster or rat liver mitochondria preparations (0.4 mg of mitochondrial protein per milliliter) in a 0.5-ml standard assay medium for 30 min. Concentrations of 5-HT and its metabolic products in the assay media then were analyzed by HPLC as described in Materials and Methods.
Rat liver mitochondrial preparations also metabolized exogenously supplied 5-HT. However, the rate of 5-HT depletion and 5-HIAA formation catalyzed by the mitochondrial preparations of the rat were remarkably different from those of the hamster. Under the same assay conditions, rat liver mitochondrial preparations metabolized 5-HT faster, yet the rate of 5-HIAA formation in rat mitochondria was much slower. Approximately 65% of the total 5-HT (10 μM) added to a rat mitochondrial preparation was metabolized in 30 min (Fig. 1B), and of that, 83% was recovered as known metabolic products with 5-HIAA being most abundant (2.4 μM), followed by 5-HIAL (2.2 μM) and 5-HTOL (0.2 μM). Approximately 17% of the 5-HT metabolized cannot be accounted for by the recovered metabolic products.
Effects of Daidzin on 5-HT Metabolism in Isolated Hamster Liver Mitochondria.
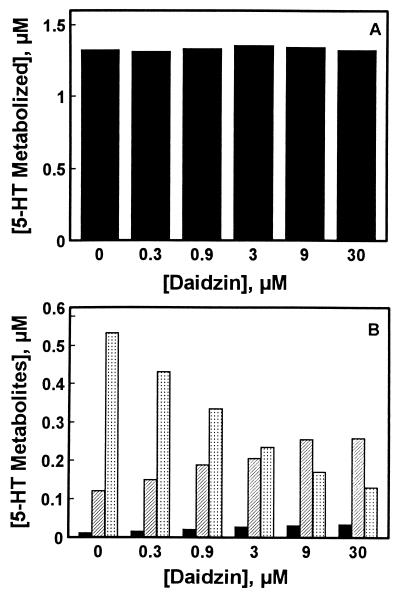
The effect of daidzin on 5-HT metabolism in hamster liver mitochondrial preparations was studied in an assay medium containing 10 μM 5-HT and 0.08 mg/ml of a mitochondrial preparation. Under these conditions, 13% (1.3 μM) of the exogenously supplied 5-HT was metabolized in 30 min (Fig. 2A), and 41, 9, and 1% of the 5-HT metabolized was converted to 5-HIAA, 5-HIAL, and 5-HTOL, respectively. Daidzin, in the concentration range studied, had no effect on the amounts of 5-HT depleted (Fig. 2A). However, it potently inhibited the formation of 5-HIAA (Fig. 2B). Inhibition was concentration-dependent, with an IC50 value of ≈2.7 μM. Inhibition of 5-HIAA formation by daidzin was accompanied by a concomitant increase in the amounts of 5-HIAL and 5-HTOL recovered in the incubation media.
Figure 2.
Effect of daidzin on 5-HT metabolism in hamster liver mitochondrial preparations. (A) 5-HT metabolized after 30 min of incubation in standard assay media containing 10 μM 5-HT and 0.08 mg/ml hamster liver mitochondrial preparation. (B) Concentrations of 5-HIAA (dotted bars), 5-HIAL (hatched bars), and 5-HTOL (solid bars) in the assay media after 30 min of incubation.
Effect of Daidzin Analogs on 5-HT Metabolism in Isolated Hamster Liver Mitochondria.
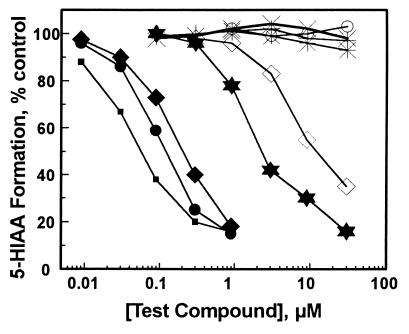
The isoflavone aglycone daidzein and its 7-O-ω-carboxyalkyl derivatives daidzein-7-O-[ω-carboxypentyl] ether (hexzein), daidzein-7-O-[ω-carboxyhexyl] ether (hepzein), and daidzein-7-O-[ω-carboxynonyl] ether (deczein) inhibited 5-HIAA formation catalyzed by intact hamster liver mitochondria in the order deczein > hepzein > hexzein > daidzin > daidzein (Fig. 3). Inhibitions were concentration-dependent, and the IC50 values estimated from these inhibition curves are listed in Table 1. Within the concentration range studied, puerarin, dicarboxymethyl-daidzein, and two structurally unrelated compounds naltrexone and γ-aminobutyric acid (GABA) had no effect on hamster liver mitochondria-catalyzed 5-HIAA formation (Fig. 3). Except for daidzein, none of the compounds examined affected the rate of 5-HT depletion in hamster liver mitochondria at concentrations that inhibited 5-HIAA formation by 50% (results not shown). Daidzein, at concentrations ≥10 μM, inhibited 5-HT depletion.
Figure 3.
Effect of daidzin and its structural analogs on 5-HT → 5-HIAA metabolism in hamster liver mitochondrial preparations. Daidzin (✶), deczein (▪), hepzein (•), hexzein (⧫), daidzein (◊), puerarin (✠), diCM-daidzein (○), naltrexone (×), and GABA (+).
Table 1.
IC50 values for daidzin and its structural analogs on the rate of 5-HIAA and DOPAC formation catalyzed by intact hamster and rat liver mitochondria
| Compounds | IC50, μM
|
|||
|---|---|---|---|---|
| Hamster
|
Rat
|
|||
| 5-HIAA | DOPAC | 5-HIAA | DOPAC | |
| Deczein | 0.06 | 0.09 | 0.05 | 0.06 |
| Hepzein | 0.13 | 0.3 | 0.15 | 0.2 |
| Hexzein | 0.21 | 0.7 | 0.5 | 0.4 |
| Daidzin | 2.7 | 2.5 | 2.0 | 2.1 |
| Daidzein | 11 | 10 | 9 | 10 |
| Puerarin | n.i. | n.i. | 30 | 28 |
| Dicarboxymethyl-daidzein | n.i. | n.i. | n.i. | n.i. |
Effects of Daidzin and Its Structural Analogs on 5-HIAA Formation in Rat Liver Mitochondria.
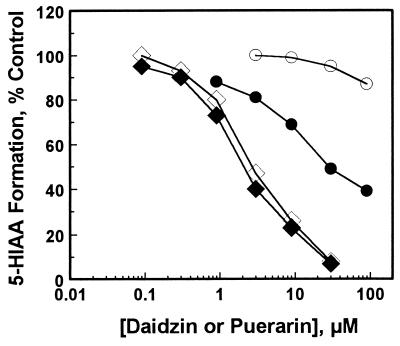
The metabolism of 5-HT → 5-HIAA in rat liver mitochondria also was inhibited by daidzin, daidzein, and the 7-O-ω-carboxyalkyl derivatives of daidzein. Inhibitions were concentration-dependent, and the IC50 values for deczein, hepzein, hexzein, daidzin, and daidzein determined under the same assay conditions were 0.05, 0.15, 0.5, 2, and 9 μM, respectively, similar to those obtained with hamster liver mitochondria (Table 1). However, the effects of puerarin on the rat and hamster liver mitochondria were markedly different. Puerarin, at concentrations up to 30 μM, had no effect on 5-HIAA formation in hamster liver mitochondria. However, over the same concentration range, it significantly inhibited 5-HIAA formation in rat liver mitochondria (Table 1). Fig. 4 shows the concentration effect of puerarin on 5-HIAA formation catalyzed by isolated rat and hamster liver mitochondria. Daidzin inhibition curves obtained from the two mitochondrial preparations used in these experiments also are shown for comparison. Dicarboxymethyl-daidzein, naltrexone, and GABA had no effect on 5-HT metabolism in rat liver mitochondria.
Figure 4.
Effect of puerarin (circles) and daidzin (diamonds) on 5-HT → 5-HIAA metabolism in rat (solid symbols) and hamster (open symbols) liver mitochondrial preparations.
Effects of Daidzin and Its Structural Analogs on DOPAC Formation in Hamster and Rat Liver Mitochondria.
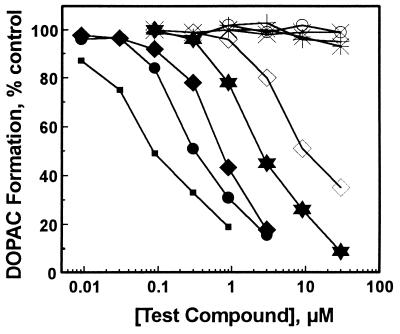
Hamster and rat liver mitochondrial preparations also metabolized exogenously supplied DA to its major metabolic product DOPAC (results not shown). Isoflavones that inhibited 5-HIAA formation also inhibited DOPAC formation in hamster liver mitochondrial preparations. Inhibitions were concentration-dependent with deczein being most potent, followed by hepzein, hexzein, daidzin, and daidzein (Fig. 5). At concentrations up to 30 μM, puerarin, dicarboxymethyl-daidzein, naltrexone, and GABA had no effect on DOPAC formation in hamster liver mitochondria.
Figure 5.
Effect of daidzin and its structural analogs on DA → DOPAC metabolism in hamster liver mitochondrial preparations. Daidzin (✶), deczein (▪), hepzein (•), hexzein (⧫), daidzein (◊), puerarin (✠), diCM-daidzein (○), naltrexone (×), and GABA (+).
Except for puerarin, DOPAC formation in rat liver mitochondria was as sensitive to the inhibition by these isoflavones as it was in hamster mitochondria (Table 1). Puerarin, at a concentration of 30 μM, had no effect on DOPAC formation catalyzed by hamster liver mitochondria. However, at the same concentration, it significantly inhibited DOPAC formation in rat liver mitochondria (results not shown).
Antidipsotropic Activity of Deczein and Dicarboxymethyl-Daidzein.
The antidipsotropic activities of deczein and dicarboxymethyl-daidzein were tested in ethanol-preferring golden hamsters, and the results are shown in Table 2. The antidipsotropic activities of five daidzin analogs tested in a previous study also are listed for comparison (12). At an i.p. dose of 70 meq/hamster/day, deczein suppressed hamster ethanol intake by 84%. At the same dose tested, hexzein, hepzein, daidzin, and daidzein suppressed hamster ethanol intake by 70, 69, 62, and 32%, respectively. Puerarin and dicarboxymethyl-daidzein did not exert any significant effect on ethanol intake in golden hamsters.
Table 2.
Inhibition of hamster liver mitochondria-catalyzed 5-HIAA and DOPAC formation and suppression of hamster ethanol intake by daidzin and its structural analogs
| Compounds | 5-HIAA formation inhibition IC50, μM | DOPAC formation inhibition IC50, μM | Ethanol intake* suppression, % | ALDH-2† inhibition Ki, μM |
|---|---|---|---|---|
| Deczein | 0.06 | 0.09 | 84 | 0.004‡ |
| Hepzein | 0.13 | 0.3 | 69‡ | 0.009‡ |
| Hexzein | 0.21 | 0.7 | 70‡ | 0.009‡ |
| Daidzin | 2.7 | 2.5 | 62‡ | 0.04‡ |
| Daidzein | 11 | 10 | 32‡ | 9‡ |
| Puerarin | n.i. | n.i. | 0‡ | 15‡ |
| Dicarboxymethyl-daidzein | n.i. | n.i. | 0 | 35 |
DISCUSSION
Oxidative deamination of monoamine neurotransmitters, catalyzed by the membrane-bound MAO on the outer surface of mitochondria, generates reactive aldehyde intermediates. These aldehydes either are oxidized to their corresponding acid metabolites by an NAD-dependent ALDH present within the mitochondrial matrix (16, 17) or are reduced to their corresponding alcohols by the cytosolic enzymes NADH-dependent ADH and/or NADPH-dependent AR (24). In the liver and brain, 5-HT and DA primarily are metabolized to their acid derivatives (25), so the mitochondrion is probably the main subcellular compartment in which this metabolism occurs in vivo. The mitochondrial preparations obtained for this study contained no detectable amounts of ADH and AR but did contain MAO and ALDH-2, the enzymes that catalyze the major pathways of 5-HT and DA metabolism. Hence, they provide a simple yet physiologically relevant system in which the effects of daidzin and its structural analogs on DA and 5-HT metabolism can be examined.
Both hamster and rat liver mitochondrial preparations were able to metabolize exogenously supplied 5-HT and DA to their respective acid metabolites 5-HIAA and DOPAC. The formation of these products was inhibited by daidzin, daidzein, and the 7-O-ω-carboxyalkyl derivatives of daidzein (Table 1). We have compared the inhibitory effects of these isoflavones on 5-HIAA or DOPAC formation in hamster liver mitochondria and hamster ethanol consumption and found a positive correlation (Table 2)—the greater the inhibition on 5-HIAA or DOPAC production, the greater the suppression of hamster ethanol consumption. These findings strongly indicate that daidzin and its antidipsotropic analogs suppress hamster ethanol intake by inhibiting 5-HT → 5-HIAA and/or DA → DOPAC metabolism.
Among the isoflavones examined, all but puerarin inhibited 5-HT → 5-HIAA and DA → DOPAC metabolism in hamster and rat mitochondria with similar potencies (Table 1). Puerarin, in the concentration range studied, had little, if any, effect on 5-HIAA and DOPAC formation in hamster liver mitochondria, but it significantly inhibited 5-HIAA and DOPAC formation in rat liver mitochondria (Table 1). Rats and golden hamsters have been shown to respond differently to puerarin in regard to their ethanol drinking behavior. Puerarin suppressed ethanol intake in P rats and Fawn Hooded rats, (4) but at equivalent doses, it had no effect in golden hamsters (12). Because both the P rats (26) and the Fawn Hooded rats (27) originated from a randomly bred Wistar colony, their 5-HT and DA metabolizing enzymes are likely to resemble those of the Wistar rats used in this study in regard to their sensitivity to puerarin inhibition. Hence, the difference in ethanol drinking response to puerarin between rats and golden hamsters may be attributed to the difference in sensitivity of their 5-HT and/or DA metabolizing enzyme(s) to puerarin inhibition. This lends additional support to the notion that daidzin may indeed suppress ethanol intake by inhibiting 5-HT → 5-HIAA and/or DA → DOPAC metabolism in the mitochondria.
In a previous study (12), we demonstrated a positive correlation between the antidipsotropic activity and ALDH-2 inhibitory activity of five isoflavones. In this study, we have added deczein and dicarboxymethyl-daidzein to the list (Table 2). Among all of the isoflavones tested, deczein is the most potent antidipsotropic agent synthesized thus far. It is also the most potent inhibitor for ALDH-2 and for mitochondria-catalyzed 5-HIAA and DOPAC formation. Dicarboxymethyl-daidzein, on the other hand, is a poor ALDH-2 inhibitor, does not inhibit mitochondria-catalyzed 5-HIAA and DOPAC formation, and does not suppress ethanol intake in golden hamsters. The fact that the inhibitory activity of these isoflavones on ALDH-2 correlates well with that on mitochondria-catalyzed 5-HIAA or DOPAC formation suggests that they may attenuate 5-HIAA or DOPAC formation in the mitochondria by inhibiting ALDH-2.
MAO and ALDH-2 act in tandem in the catalytic conversion of 5-HT and DA to their respective acid metabolites 5-HIAA and DOPAC. Therefore, in principle, daidzin and its antidipsotropic analogs also could block 5-HIAA/DOPAC formation by inhibiting MAO. To evaluate this possibility, we have studied the effect of these isoflavones on MAO by using partially purified mitochondrial membranes. Daidzin, puerarin, and dicarboxymethyl-daidzein, at concentrations up to 30 μM, exhibited no effect on the MAO activity of the mitochondrial membranes. The 7-O-ω-carboxyalkyl derivatives of daidzein do inhibit MAO activity but only at high concentrations (Table 3). These results are consistent with the finding that daidzin (Fig. 2A) and its antidipsotropic analogs, at concentrations that significantly inhibited 5-HIAA or DOPAC formation, have no effect on 5-HT and DA depletion. Furthermore, MAO inhibitory activities of the isoflavones examined do not correlate with their antidipsotropic activities (Table 3). It appears that the antidipsotropic action of daidzin, and presumably that of its antidipsotropic analogs as well, is not mediated by 5-HT and/or DA but rather by their reactive intermediates 5-HIAL and/or DOPAL, which accumulate under the action of daidzin (Fig. 2B).
Table 3.
Effect of daidzin and its structural analogs on hamster liver MAO activity
| Compounds | MAO inhibition IC50, μM | Ethanol intake* suppression, % |
|---|---|---|
| Deczein | 25 | 84 |
| Hepzein | 9 | 69† |
| Hexzein | 10 | 70† |
| Daidzin | n.i. | 62† |
| Daidzein | 22 | 32† |
| Puerarin | n.i. | 0† |
| Dicarboxymethyl-daidzein | n.i. | 0 |
Hamster liver mitochondrial membrane was used as a source of MAO. MAO activity was assayed as described in Materials and Methods by using 10 μM DA as the substrate. n.i, no inhibition up to 30 μM.
Ethanol intake-suppresssive activity was measured as described in ref. 1. Dose = 70 meq per hamster per day, i.p.
Data taken from ref. 12.
In the mitochondrial preparations, concentrations of 5-HIAL attained during 5-HT metabolism are determined by the relative catalytic efficiency of MAO and ALDH-2. For instance, rat liver mitochondrial preparations have a much higher MAO-to-ALDH-2 activity ratio than that of hamster (1.6 vs. 0.18), and as a consequence, 5-HIAL concentrations found in the former are also much higher than in the latter (Fig. 1). In this context, it is of interest to note that golden hamsters are by nature inclined to prefer and consume large quantities of ethanol (28) whereas the randomly bred Wistar rats used in this study tend to avoid ethanol (26). Epidemiological studies also have associated low MAO and/or high ALDH-2 activities with high ethanol consumption: (i) low platelet MAO activity correlates with type II alcoholism (29), and (ii) Asians who have inherited a low activity (or inactive) mutant form of ALDH-2 are protected from the problem of alcohol abuse (10). These findings by no means prove, but are consistent with, the hypothesis that a metabolic intermediate of 5-HT, and presumably of DA also, may be involved in the regulation of ethanol drinking.
5-HIAL, and other biogenic aldehydes as well, are very reactive compounds. Difficulties encountered in synthesizing and monitoring these aldehydes have greatly hampered detailed studies on their metabolism and potential roles in neuronal and other physiological processes. Early interest in biogenic aldehydes in relation to alcohol research stems largely from the belief that acetaldehyde, the metabolic intermediate of ethanol, interferes in some way with the oxidative metabolism of the brain (30, 31). It was hypothesized that levels of biogenic aldehydes increase during ethanol metabolism because of competitive inhibition of ALDH by acetaldehyde. This could cause a shift in the metabolism of these biogenic aldehydes, such as 5-HIAL and DOPAL, toward the reductive pathway that leads to the formation of 5-HTOL and DOPET, respectively. The physiological implication, if any, of shifting from an oxidative to a reductive metabolic pathway is completely unknown at this time.
Increased levels of biogenic aldehydes could promote the formation of alkaloid condensation products between the biogenic aldehydes and their parent amines or other endogenous or exogenous amines (32). One of these condensation products, tetrahydropapaveroline formed between DA and DOPAL, is a morphine precursor found in the opium poppy and may have potential effects on opiate and ethanol addiction (33). Biogenic aldehydes themselves are physiologically active and may play an essential role in mediating the intake and actions of ethanol. 5-HIAL has been shown to induce sleep in newly hatched chicks (34), inhibit brain ATPase (35), affect visually evoked responses in rabbits (36), bind to neuronal membranes both in vitro and in vivo (37), and depress the firing rate of single neurons in the prefrontal cortex and cerebellum (38). Unfortunately, none of these biological activities of 5-HIAL and/or DOPAL has been investigated in depth. The potential role of biogenic aldehydes in regulating ethanol drinking behavior remains to be explored.
Acknowledgments
We thank Dr. O. Lazo for assistance in the early phase of this work, Dr. D.-J. Li for assistance in the animal drinking experiments, Dr. Nedège Lagneau for synthesizing deczein and dicarboxymethyl-daidzein, and Drs. J. F. Riordan and T. C. French for valuable discussions. This work was supported by the Endowment for Research in Human Biology.
ABBREVIATIONS
- ALDH
aldehyde dehydrogenase
- ADH
alcohol dehydrogenase
- MAO
monoamine oxidase
- AR
aldehyde reductase
- 5-HT
serotonin
- 5-HIAA
5-hydroxyindole-3-acetic acid
- 5-HIAL
5-hydroxyindole-3-acetaldehyde
- 5-HTOL
5-hydroxytryptophol
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- DOPET
3,4-dihydroxyphenylethanol
- GABA
γ-aminobutyric acid
- deczein
daidzein-7-O-[ω-carboxynonyl] ether
- hepzein
daidzein-7-O-[ω-carboxyhexyl] ether
- hexzein
daidzein-7-O-[ω-carboxypentyl] ether
References
- 1.Keung W M, Vallee B L. Proc Natl Acad Sci USA. 1993;90:10008–10012. doi: 10.1073/pnas.90.21.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keung W M, Vallee B L. EXS. 1994;71:1254–1260. doi: 10.1007/978-3-0348-7330-7_37. [DOI] [PubMed] [Google Scholar]
- 3.Heyman G M, Keung W M, Vallee B L. Alcohol Clin Exp Res. 1996;20:1083–1087. doi: 10.1111/j.1530-0277.1996.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 4.Overstreet D H, Lee Y W, Rezvani A H, Pei Y H, Criswell H E, Janowsky D S. Alcohol Clin Exp Res. 1996;20:221–227. doi: 10.1111/j.1530-0277.1996.tb01633.x. [DOI] [PubMed] [Google Scholar]
- 5.Overstreet D H, Rezvani A H, Lee Y W. Alcohol Clin Exp Res. 1996;20:16A. doi: 10.1111/j.1530-0277.1996.tb01633.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin R C, Guthrie S, Xie C-Y, Mai K, Lee D Y, Lumeng L, Li T-K. Alcohol Clin Exp Res. 1996;20:659–663. doi: 10.1111/j.1530-0277.1996.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 7.Keung W M, Vallee B L. Proc Natl Acad Sci USA. 1993;90:1247–1251. doi: 10.1073/pnas.90.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tipton K F, Henehan G T, Harrington M C. In: Human Metabolism of Alcohol: Regulation, Enzymology, and Metabolites of Ethanol. Crow K E, Batt R C, editors. Vol. 2. Boca Raton, FL: CRC; 1989. pp. 105–116. [Google Scholar]
- 9.Harada S, Agarwal D P, Goedde H W. Lancet. 1981;2:982. doi: 10.1016/s0140-6736(81)91172-7. [DOI] [PubMed] [Google Scholar]
- 10.Harada S, Agarwal D P, Goedde H W, Tagaki S, Ishikawa B. Lancet. 1982;2:827. doi: 10.1016/s0140-6736(82)92722-2. [DOI] [PubMed] [Google Scholar]
- 11.Ohmori T, Koyama T, Chen C-C, Yeh E-K, Reyes B V, Yamachita I. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:229–235. doi: 10.1016/0278-5846(86)90077-1. [DOI] [PubMed] [Google Scholar]
- 12.Keung W M, Klyosov A A, Vallee B L. Proc Natl Acad Sci USA. 1997;94:1675–1679. doi: 10.1073/pnas.94.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keung W M, Lazo O, Kunze L, Vallee B L. Proc Natl Acad Sci USA. 1995;92:8990–8993. doi: 10.1073/pnas.92.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelrod J, Kopin I J, Mann J D. Biochim Biophys Acta. 1959;36:576–585. doi: 10.1016/0006-3002(59)90218-5. [DOI] [PubMed] [Google Scholar]
- 15.Erwin V G, Deitrich R A. J Biol Chem. 1966;241:3533–3539. [PubMed] [Google Scholar]
- 16.Tank A W, Weiner H, Thurman J A. Biochem Pharmacol. 1981;30:3265–3275. doi: 10.1016/0006-2952(81)90598-0. [DOI] [PubMed] [Google Scholar]
- 17.Ambroziak W, Pietruszko R. J Biol Chem. 1991;266:13011–13018. [PubMed] [Google Scholar]
- 18.Iyer R N, Shah K H, Venkataraman K. Proc Indian Acad Sci. 1951;33:116–126. [Google Scholar]
- 19.Farka L, Varady J. Ber Dtsch Chem Ges. 1959;92:819–821. [Google Scholar]
- 20.Nilsson G E, Tottmar O. J Neurochem. 1987;48:1566–1572. doi: 10.1111/j.1471-4159.1987.tb05702.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson D, Lardy H. Methods Enzymol. 1967;10:94. [Google Scholar]
- 22.Beaufay H, Bendall D S, Baudhuin P, deDuve C. J Cell Biol. 1959;73:623–628. doi: 10.1042/bj0730623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fong W P, Keung W M. Hum Hered. 1989;39:185–191. doi: 10.1159/000153858. [DOI] [PubMed] [Google Scholar]
- 24.Feldstein A, Wong K K-K. Life Sci. 1964;4:183–191. doi: 10.1016/0024-3205(65)90118-9. [DOI] [PubMed] [Google Scholar]
- 25.Feldstein A. In: The Biology of Alcoholism. Kissin B, Beleiter H, editors. New York: Plenum; 1971. pp. 127–159. [Google Scholar]
- 26.Li T-K, Lumeng L, Mcbride W J, Waller M B. Drug Alcohol Depend. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, Aulakh C S, Hill J L, Murphy D L. Psychopharmacology. 1988;94:558–562. doi: 10.1007/BF00212855. [DOI] [PubMed] [Google Scholar]
- 28.Arvola A, Forsander O. Nature (London) 1961;191:819–820. doi: 10.1038/191819a0. [DOI] [PubMed] [Google Scholar]
- 29.von Knorring A-L, Bohman M, von Knorring L, Oreland L. Acta Psychiatr Scand. 1985;72:51–58. doi: 10.1111/j.1600-0447.1985.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 30.Beer C T, Quastel J H. Can J Biochem Physiol. 1958;36:531–541. [PubMed] [Google Scholar]
- 31.Truitt E B, Bell F K, Krantz J C. Q J Stud Alcohol. 1956;17:594. [PubMed] [Google Scholar]
- 32.Deitrich R A, Erwin V G. Fed Proc. 1975;34:1962–1968. [PubMed] [Google Scholar]
- 33.Goldstein D B, Judson B A. Science. 1971;172:290–293. doi: 10.1126/science.172.3980.290. [DOI] [PubMed] [Google Scholar]
- 34.Sabelli H C, Giardina W J. Arzneimittelforsch. 1970;20:68–74. [Google Scholar]
- 35.Tabakoff B. Res Commun Chem Pathol Pharmacol. 1974;7:621–624. [PubMed] [Google Scholar]
- 36.Sabelli H C, Giardina W J, Alivisatos S G A, Seith P K, Ungar F. Nature (London) 1969;223:73–74. doi: 10.1038/223073a0. [DOI] [PubMed] [Google Scholar]
- 37.Alivisatos S G A, Tabakoff B. In: Chemical Modulation of Brain Function. Sabelli H, editor. New York: Raven; 1973. [Google Scholar]
- 38.Palmer M R, Tottmar O, Deitrich R A. Alcohol Clin Exp Res. 1986;10:682–685. doi: 10.1111/j.1530-0277.1986.tb05168.x. [DOI] [PubMed] [Google Scholar]