Abstract
GTPase-activating proteins (GAPs) function by stabilizing the GTPase transition state. This has been most clearly demonstrated by the formation of a high-affinity complex between various GAPs and GDP-bound GTPases in the presence of aluminum tetrafluoride, which can mimic the γ-phosphate of GTP. Herein, we report that p190 RhoGAP forms a high-affinity complex with Rho GTPases in the presence of fluoride ions, suggesting that p190 also functions to stabilize the GTPase transition state. However, this Rho–p190 complex does not require aluminum ions or even guanine nucleotide, indicating a distinct role for fluoride that is not consistent with the γ-phosphate-mimicking hypothesis. These results indicate that it is necessary to reconsider the assumed role of fluoride in stabilizing a variety of other GTPase–GAP interactions where the requirement for aluminum or guanine nucleotide has not yet been addressed.
The observation that aluminum fluoride can bind to and activate heterotrimeric G proteins has proven to be tremendously useful for the study of G protein activation in vivo, for the elucidation of three-dimensional structures of several GTPases, and for understanding the biochemical mechanism of GTP hydrolysis, including the role of GTPase-activating proteins (GAPs) (1). Aluminum fluoride appears to function as a G protein activator by interacting with the GDP-bound Gα subunits of heterotrimeric G proteins in such a way as to mimic the γ-phosphate of GTP, thereby promoting formation of the GTPase transition state (2). Although it was initially believed that AlF4− only interacts with Gα subunits (3), recent studies have revealed that the small GTPase Ras can interact stably with AlF3 in the presence of RasGAP (4). Subsequently, similar studies demonstrated that several classes of small GTPases can stably interact with their respective GAPs in the presence of AlF3 (5), suggesting that aluminum fluoride molecules can bind to a wide variety of GTPases under conditions that favor formation of the GTPase transition state. Moreover, these observations are consistent with an earlier hypothesis that the GTP hydrolysis mechanism is similar for both small GTPases and Gα subunits and that GAPs for the small GTPases might function by stabilizing the GTPase transition state (6).
In the studies described herein, the ability of fluoride to promote a high-affinity complex between the Ras-related RhoA GTPase and the p190 RhoGAP is reported. Surprisingly, however, formation of this high-affinity complex does not require either aluminum or guanine nucleotide, suggesting a role for fluoride distinct from the γ-phosphate-mimicking role that has been previously described. These results and the fact that the specific requirement for aluminum or guanine nucleotide has not been tested in numerous other previously reported aluminum fluoride-promoted GTPase–GAP interactions suggest that it is necessary to reexamine the role of fluoride in such complexes.
MATERIALS AND METHODS
Preparation of Recombinant Proteins.
Human RhoA, RhoAV14, Rac1, and Ha-Ras GTPases expressed as glutathione S-transferase (GST) fusion proteins were prepared from bacteria or baculovirus-infected sf9 cells (for RhoA only) after lysis in 10 ml of a solution containing 20 mM Tris⋅HCl (pH 7.5), 50 mM NaCl, 2.5 mM MgCl2, 1 mM DTT, 0.1 mM GDP, 0.7% Triton X-100, aprotinin (10 μg/ml), leupeptin (10 μg/ml), and 1 mM phenylmethylsulfonyl fluoride. Lysates were sonicated for four 20-sec periods and centrifuged at 10,000 × g for 20 min, and supernatants were incubated with 1 ml of a 60% slurry of glutathione-agarose beads (Sigma) for 3 h at 4°C. After extensive washing of the beads in lysis buffer, fusion proteins were eluted in a solution containing 20 mM Tris⋅HCl (pH 7.5), 200 mM NaCl, 2.5 mM MgCl2, 1 mM DTT, 0.1 mM GDP, and 0.01% Triton X-100 in the presence of 10 mM glutathione at room temperature; dialyzed overnight in a solution of 20 mM Tris⋅HCl (pH 7.5), 50 mM NaCl, 2.5 mM MgCl2, 1 mM DTT, 0.01% Triton X-100, and 20% glycerol; and stored in aliquots at −80°C. Baculovirus-produced p190 was prepared as described (7).
Cell Culture and Transfection.
Fibroblasts cell lines 79–5 and 79–3 were derived from wild-type mouse embryos or embryos genetically modified to express a GTPase-deleted version of p190 RhoGAP, respectively (to be described elsewhere). Cos-7 cells and mouse fibroblasts 79–5 and 79–3, and Swiss 3T3 were maintained under standard conditions. Cos-7 cells in 10-cm dishes were transfected with 10 μg of expression plasmids pRcHA-p190wt (8) or pRcHA-30–1 (9) by the dextran sulfate method. Cell extracts were prepared in lysis buffer [50 mM Hepes, pH 7.2/150 mM NaCl/1.5 mM MgCl2/5 mM EGTA/10% glycerol/1% Triton X-100/aprotinin (10 μg/ml)/leupeptin (10 μg/ml)/1 mM phenylmethylsulfonyl fluoride] 48 h after transfection (for Cos-7).
GTPase Binding Assays.
Typically, for analysis of six samples, 3–6 μg of GST fusion protein was incubated with 60 μl of glutathione-agarose beads for 30 min at room temperature and subjected to nucleotide exchange either in the absence of nucleotide or in the presence of 1 mM GDP or guanosine 5′-[γ-thio]triphosphate in a buffer containing 5 mM EDTA, as described (9). After a 15-min incubation at 37°C, the exchange reaction was stopped by adding 20 mM MgCl2. For GTPase binding assay, extracts from either fibroblasts or baculovirus p190-infected sf9 cells were incubated with the beads for 1 h at 4°C. After washing the beads three times with 1 ml of washing buffer (20 mM Hepes, pH 7.2/150 mM NaCl/1.5 mM MgCl2/10% glycerol/0.1% Triton X-100), bound proteins were analyzed by SDS/PAGE on 7.5% gels and immunoblotting with antibodies directed against p190 (D2D6 monoclonal antibody), p190-B (polyclonal antiserum that does not cross-react with p190), the hemagglutinin epitope tag (12CA5 monoclonal antibody), or bacterial GST (A2 monoclonal antibody). Deferoxamine (Sigma) was included at 0.5 mM and EGTA was included at 5 mM where indicated. For experiments with highly purified p190, baculovirus-produced p190 was isolated from infected sf9 cells as described (7). For the nucleotide-depletion experiment, GST-RhoA bound to beads was first loaded with 10 μCi of [α-32P]GDP (1 Ci = 37 GBq), and the beads were then washed to remove free nucleotide and subjected to an exchange reaction in absence of nucleotide. Aliquots of this reaction were removed at several times and mixed with 1 ml of a ice-cold buffer containing 5 mM MgCl2. Proteins were spotted onto 0.45-μm (pore size) nitrocellulose filters (Schleicher & Schuell), which were then washed twice with 20 mM Tris⋅HCl, pH 7.2/50 mM NaCl/5 mM MgCl2/1 mM DTT, and filter-bound radioactivity was measured by Cerenkov counting.
RESULTS
Fluoride Promotes Formation of a High-Affinity Complex Between the Rho GTPase and p190.
p190 RhoGAP exhibits a specific GAP activity toward members of the Rho GTPase family (10). Previously, it had been reported that the RhoA GTPase forms a stable complex with p190 in the presence of sodium orthovanadate and sodium fluoride (NaF), when added as phosphatase inhibitors (11). We extended this observation to determine whether these compounds affect the Rho–p190 interaction directly or act indirectly by affecting p190 phosphorylation. For these experiments, a purified recombinant GST fusion protein of the RhoA GTPase expressed in bacteria was immobilized on glutathione-agarose beads, loaded with guanine nucleotides, and incubated with fibroblast cell lysate. Stable binding of p190 to immobilized GST-RhoA was then assayed by immunoblotting with p190-specific antibodies. As shown in Fig. 1A, GDP-loaded RhoA binds stably to p190 specifically when NaF (20 mM), but not sodium orthovanadate, is added to the mixture. A variety of other tested anions failed to promote the RhoA–p190 association (data not shown).
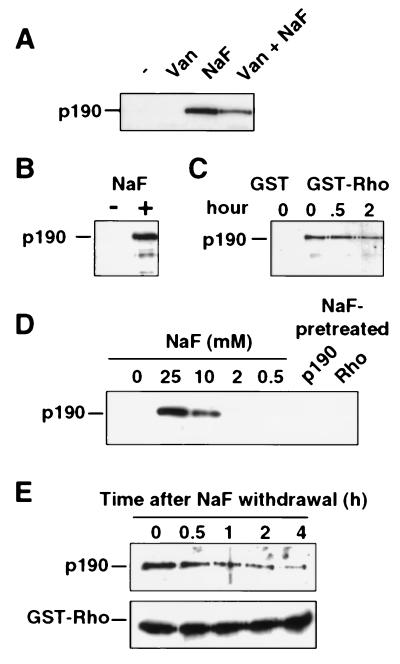
Figure 1.
Fluoride stabilizes a high-affinity complex between the RhoA GTPase and p190 RhoGAP. Recombinant GST-RhoA protein (GDP-loaded) immobilized on glutathione beads was used in a p190-binding assay with either fibroblast extract (A and C) or recombinant baculovirus-produced p190 (B, D, and E). After 1 h of incubation, beads were washed three times and complex formation with p190 was analyzed by SDS/PAGE and immunoblotting using an anti-p190 antibody or an anti-GST-specific antibody. (A) The binding reaction was either unsupplemented (−) or supplemented with 1 mM sodium orthovanadate (Van), 20 mM NaF, or both (Van+NaF), as indicated. (B) Highly purified baculovirus-produced p190 was incubated with GST-RhoA in the presence or absence of 20 mM NaF. (C) Cell extracts lysed in the absence of NaF were incubated at 37°C for 0, 0.5, or 2 h before incubation with GST or GST-RhoA in the presence of NaF for 1 h at 4°C. (D) Similar p190–Rho binding assays were performed in the presence of a range of NaF concentrations (as indicated). In lanes p190 and Rho, p190 or RhoA were pretreated for 1 h with 20 mM NaF before a binding assay in which the final concentration of NaF was reduced to 2 mM NaF. (E Upper) The RhoA–p190 complex was formed in the presence of 20 mM NaF, and the washed beads were incubated in NaF-free buffer for the indicated times and then assayed for retention of p190. (E Lower) An anti-GST antibody reveals equivalent amounts of GST-RhoA.
To determine whether the observed effects of NaF are due to inhibition of serine/threonine phosphatase activity, two experiments were performed. First, we demonstrated that highly purified recombinant p190 (7) also binds tightly to GST-RhoA in the presence, but not in the absence, of NaF (Fig. 1B). If phosphatase activity is indeed required to prevent RhoA-p190 binding, then this phosphatase is apparently effective during a 1-h incubation at 4°C, where no RhoA–p190 complex is seen in the absence of NaF (Fig. 1 A and B). Therefore, we next examined the ability of fluoride to promote RhoA–p190 association in fibroblast extracts that were incubated for as long as 2 h at 37°C before the addition of NaF. This should provide more than enough time for any potential phosphatase activity to be effective. However, no reduction in NaF-promoted RhoA–p190 complex formation is seen when the NaF is added after such incubation (Fig. 1C). The same result was obtained when purified p190 was incubated at 37°C prior to NaF addition (data not shown). Thus, these results indicate that fluoride is not functioning as a phosphatase inhibitor in these binding assays. Subsequent binding experiments were performed with insect cell lysate containing baculovirus-produced p190 (7). Stable RhoA–p190 binding was found to be dependent on the NaF concentration and binding is approximately half-maximal at 10 mM NaF (Fig. 1D). By preincubating p190 or RhoA with 20 mM NaF for 1 h before coincubation at a final NaF concentration below that required for complex formation (2 mM), we were able to demonstrate that fluoride must be present at a sufficient concentration during complex formation, suggesting that fluoride participates directly in the stable complex (Fig. 1D). In addition, the stability of the fluoride-promoted RhoA–p190 complex was further demonstrated by examining the duration of the complex after withdrawal of NaF (Fig. 1E). The half-life of the complex appears to be 1–2 h, indicating that the complex dissociates relatively slowly, once formed. Reducing either the amount of GST-RhoA or p190 in the binding assay results in a proportional decrease in complex formation, indicating that the stochiometry of the complex is probably 1:1 (Fig. 4C and data not shown).
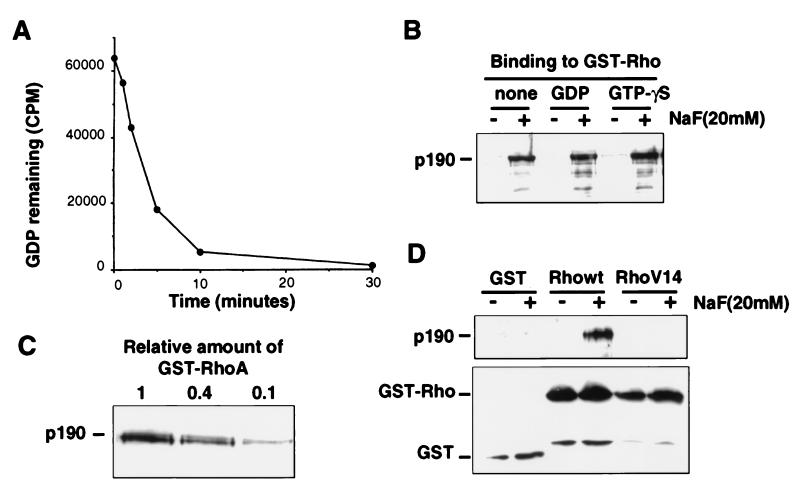
Figure 4.
Fluoride-promoted RhoA–p190 complex is nucleotide-dependent but requires a GTPase-competent RhoA. (A) [α-32P]GDP-loaded RhoA protein was incubated in buffer containing 5 mM EDTA, and aliquots were removed at the indicated times and analyzed by filter binding to demonstrate the release of bound GDP. (B) Interaction of baculovirus-produced p190 with nucleotide-depleted or GDP- or guanosine 5′-[γ-thio]triphosphate-loaded GST-RhoA on beads was examined in the presence or absence of NaF and bound p190 was detected by immunoblotting with anti-p190 antibody. (C) Various amounts of GST-RhoA (GDP-loaded) were incubated with p190 in the presence of 20 mM NaF, and bound p190 was detected by immunoblotting. The value 1 is an amount of RhoA equivalent to that used in all preceding assays. (D Upper) Binding of p190 to wild-type RhoA and the constitutively active form of Rho, RhoV14, was compared in the presence or absence of NaF and detected by anti-p190 immunoblotting. (D Lower) Immunoblotting with anti-GST antibodies revealed that approximately equivalent amounts of GTPase fusion protein were used.
We next examined the specificity of the fluoride-promoted RhoA–p190 complex. When the ability of p190 to bind stably to GST-Ras, GST-RhoA, and GST-Rac1 was compared in the presence or absence of NaF, p190 exhibited a stable NaF-dependent association with RhoA and Rac1 but not with Ras (Fig. 2A). This is consistent with the previously reported ability of p190 to function as a GAP for Rac1 and RhoA (10) and indicates that the observed stable binding to p190 is not RhoA-specific but is specific to normal targets for p190. The lower efficiency of p190 complex formation with Rac compared with RhoA parallels the relative GAP activity exhibited by p190 toward these two GTPases (12) and suggests that these complexes reflect the normal GTPase–GAP interaction, as confirmed below. Recently, a closely related p190 family member, p190-B, was identified, that also exhibits RhoGAP activity (13). We found that p190-B, like p190, exhibits a high-affinity interaction with RhoA in the presence of NaF (Fig. 2B). Additional evidence of binding specificity was obtained with the demonstration that GST-RhoA selectively captures p190 from a total cellular extract of insect cells infected with a p190 baculovirus when fluoride is provided (Fig. 2C). Thus, these results indicate that p190 proteins are able to bind with high affinity to their target GTPases specifically in the presence of fluoride ions.
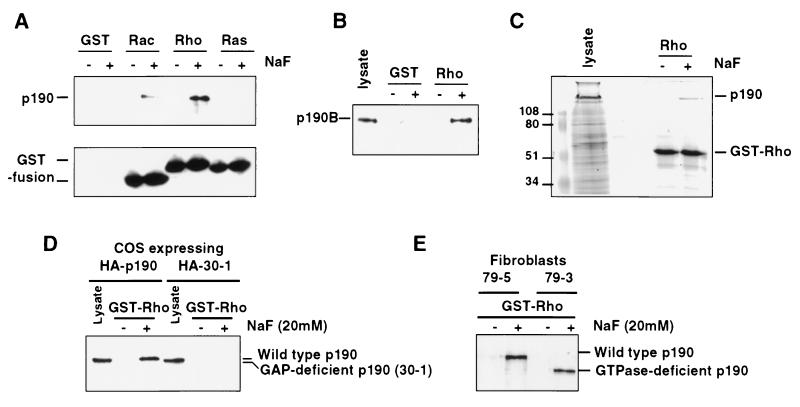
Figure 2.
High-affinity fluoride-promoted RhoA–p190 complex requires a functional p190 RhoGAP domain. (A) GTPase binding assays were performed with baculovirus p190 using recombinant GST, GST-Rac, -Rho, or -Ras proteins. p190 (Upper) and GST fusion proteins (Lower) were detected by immunoblotting. Unfused GST protein is not seen due to its low molecular weight. (B) Association of p190-B (from extracts of Swiss 3T3 cells) with RhoA is also fluoride-dependent as revealed by GST-RhoA binding assays. (C) Coomassie blue staining of an SDS/PAGE gel of crude baculovirus-infected cell lysate overexpressing p190 and GST-RhoA association with p190 specifically in the presence of NaF. (D) Cos-7 cells were transfected with vectors expressing a hemagglutinin-tagged form of either wild-type p190 (HA-p190) or p190 deleted of the RhoGAP domain (HA-30–1), and lysates were subjected to a RhoA binding assay in the presence or absence of NaF. Expression (lysates) and GST-RhoA-associated p190 were detected with anti-hemagglutinin antibodies. (E) Lysates from fibroblasts expressing either wild-type p190 (79–5) or a GTPase-deleted form of p190 (79–3) were subjected to a similar RhoA binding assay.
The Fluoride-Promoted RhoA–p190 Complex Requires the RhoGAP Domain of p190.
To determine whether the observed association between RhoA and p190 is mediated by the RhoGAP catalytic domain of p190, we expressed, in transfected Cos cells, epitope-tagged versions of wild-type p190 and a p190 deletion mutant (30–1) that removes 48 amino acids from the catalytic domain (amino acids 1,395–1,443) and is completely defective for RhoGAP activity. Cell lysates were incubated with RhoA beads in the presence of NaF and stably associated p190 was detected by immunoblotting with the anti-tag antibody. As shown (Fig. 2D), wild-type p190, but not the deletion mutant, could be detected in a stable complex with RhoA in the presence of NaF. These results indicate that the observed interaction requires the RhoGAP catalytic domain of p190. Because p190 RhoGAP also contains an amino-terminal GTPase domain (14), we examined the role of this region of the protein in the fluoride-dependent complex with RhoA. By using an amino-terminal deletion mutant of p190 that specifically removes the GTPase domain, we were able to demonstrate that this region of the protein is not required for the fluoride-dependent RhoA–p190 interaction (Fig. 2E). Thus, it appears likely that fluoride ions are able to influence the normal interaction of the RhoGAP region of p190 with its target GTPases.
The Fluoride-Promoted RhoA–p190 Complex Does Not Involve Aluminum Fluoride.
GAPs for the small GTPases and for the Gα subunits of heterotrimeric G proteins [regulators of G protein signaling (RGS)] have recently been shown to be capable of stabilizing the transition state of GTPases in the presence of aluminum fluoride (4, 5). Significantly, however, we found that NaF, in the absence of exogenously added aluminum, appears to enable p190 to stabilize the transition state of the RhoA GTPase. Because a previous report revealed that micromolar aluminum concentrations can often be detected as a contaminant of laboratory glassware (15), we addressed the requirement for aluminum in two experiments. In the first experiment, we examined the ability of the chelating agents, EGTA and deferoxamine, which can bind aluminum ions, to affect the NaF-dependent RhoA–p190 complex. Deferoxamine has been used extensively in previous studies to demonstrate a requirement for aluminum in the aluminum fluoride activation of several G proteins (16–19). As shown in Fig. 3A, these chelators do not detectably affect the observed NaF-dependent RhoA–p190 complex. In the second experiment, AlCl3 (100 μM) was added to the incubation mixture in the presence of suboptimal NaF concentration (5 mM) to look for an enhanced complex formation. As shown (Fig. 3A), no effect of AlCl3 was observed. Thus, these results suggest that fluoride ions, in the absence of an aluminum fluoride complex, can stabilize the RhoA–p190 interaction.
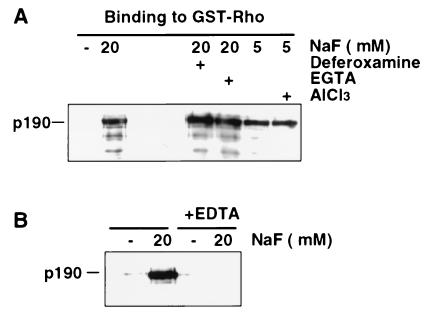
Figure 3.
High-affinity fluoride-promoted RhoA–p190 complex is aluminum-independent but requires magnesium. (A) Baculovirus p190 was incubated with GST-RhoA beads in the presence of NaF at the indicated concentrations and either the aluminum chelators deferoxamine (0.5 mM) or EGTA (5 mM) or AlCl3 (100 μM), as indicated. (B) The RhoA–p190 binding assay was performed in the presence or absence of EDTA (5 mM). p190 was detected by immunoblotting after SDS/PAGE.
A few previous reports have identified a similar aluminum-independent effect of fluoride on G protein activation (20, 21). Because those studies suggest that such activation involves the formation of a Mg2F3 ion, we examined the requirement for Mg2+ by using the chelator EDTA at 5 mM, which should effectively reduce the free Mg2+ concentration to less than 5 μM under these assay conditions. As shown (Fig. 3B), EDTA completely eliminates the NaF-induced RhoA–p190 complex. Thus, these results are consistent with a molecular mechanism for Mg2+ and F− similar to that previously described for the aluminum-independent activation of Gα proteins.
The Fluoride-Promoted RhoA–p190 Complex Does Not Require Guanine Nucleotide.
In conducting the Mg2+ requirement experiments described above, we found that when Mg2+ was added back to RhoA protein after exposure to EDTA, the NaF-induced RhoA–p190 interaction could be restored (data not shown). EDTA, at the concentration used in this experiment, is known to facilitate the rapid release of guanine nucleotide from GTPases (22), suggesting that the NaF-promoted RhoA–p190 interaction may not require guanine nucleotide. To test this possibility, we first incubated RhoA with EDTA-containing buffer for 60 min at 37°C in the absence of additional guanine nucleotide to release any bound nucleotide. The RhoA protein bound to beads was then washed twice in the same buffer to completely remove any released nucleotide. Similar treatments have been shown to promote the rapid release of nucleotide from RhoA (23). To demonstrate that this treatment effectively removes bound nucleotide, we prepared [32P]GDP-loaded RhoA in a standard nucleotide exchange reaction, then incubated the protein in the EDTA-containing buffer for 30 min, and removed aliquots at various times to follow the release of labeled nucleotide in a filter-binding assay. As shown (Fig. 4A), more than 98% of bound nucleotide is released from RhoA after 30 min under these conditions. Nucleotide-depleted RhoA (after 60 min in EDTA) was tested for p190 association in the presence or absence of NaF at magnesium concentration of 5 mM. As shown (Fig. 4B), nucleotide-depleted RhoA is able to bind p190 stably in a NaF-dependent manner as efficiently as GDP-loaded or guanosine 5′-[γ-thio]triphosphate-loaded RhoA. To demonstrate that the observed “nucleotide-free” binding is not due to the ability of a very small amount of remaining GDP-bound RhoA to complex with p190, we determined that reducing the amount of RhoA–GDP in our standard p190-binding assay by as little as 2.5-fold significantly decreases the amount of detectable fluoride-promoted RhoA–p190 complex (Fig. 4C). Thus, these results suggest that fluoride is playing a role in RhoA–p190 complex stabilization that is clearly distinct from the γ-phosphate-mimicking role that has been previously documented for other GTPases.
p190 Appears to Function by Stabilizing the Transition State of the Rho GTPase.
Several single amino acid substitutions of the RhoA GTPase have been described that disrupt GTP hydrolytic function and confer resistance to GAP activity (10, 24). We used one of these, RhoAV14, to determine whether this defect in catalysis might be due to an inability of such mutants to assume the normal transition state. As shown (Fig. 4D), unlike wild-type RhoA, a similar preparation of RhoAV14 is unable to associate stably with p190 in the presence of NaF and Mg2+. As expected, we found that the interaction of RhoA with protein kinase N, a Rho effector target (25, 26) that would not be predicted to induce the transition state of RhoA, is unaffected by fluoride (data not shown). Thus, these results support the hypothesis that p190 is playing a role in stabilizing the Rho GTPase transition state and that this RhoA mutant is resistant to GAP activity because it is unable to undergo a conformational change required to enter the transition state. Similarly, p50 RhoGAP fails to stabilize the transition state of the analogous CDC42V12 mutant in the presence of aluminum fluoride (5).
DISCUSSION
We have demonstrated that p190 RhoGAP can form a high-affinity complex with the RhoA GTPase in a fluoride- and magnesium-dependent but aluminum- and guanine nucleotide-independent manner. Previous studies of the aluminum fluoride-promoted Ras–RasGAP and Gα-RGS protein interactions revealed that the likely role for aluminum fluoride in the ability of these GAPs to stabilize the transition state of GDP-bound GTPases is to mimic the γ-phosphate of GTP. This appears to involve the formation of an octahedrally coordinated Al3+ ion with a geometry similar to that of the pentacovalent phosphorous intermediate in the GTPase reaction (4, 27). Thus, analogous to results recently reported in studies of the Ras–RasGAP interaction, our results are consistent with a mechanism wherein p190 RhoGAP promotes a conformation of RhoA that allows RhoA to bind fluoride and become stabilized in its transition state. This was indeed confirmed by using the GTP-hydrolysis-defective RhoAV14 mutant.
The absence of a requirement for aluminum or guanine nucleotide in the fluoride-mediated RhoA–p190 complex was surprising and contradicts the widely held γ-phosphate mimicking hypothesis of GTPase activation by fluoride. This result was further substantiated with the demonstration that fluoride promotes the interaction of p190 with guanosine 5′-[γ-thio]triphosphate-loaded RhoA as efficiently as GDP-loaded RhoA. In addition, the fact that p190 can apparently stabilize the transition state of the nucleotide-free Rho GTPase suggests that the RhoA GTPase structure is largely maintained in the absence of guanine nucleotide and that the nucleotide may not be required for recognition of the Rho structure by the p190 protein.
Although interactions between several small GTPases (Ras, Ran, Rap, and CDC42) and their respective GAPs are also stabilized by fluoride (5), a specific requirement for aluminum or guanine nucleotide has not been examined in those cases. In addition, high-affinity stable interactions have been demonstrated between some of the GAP-like RGS proteins and their Gα targets in the presence of AlF4 (28–33). Because a specific requirement for aluminum or guanine nucleotide has also not been examined in those interactions, it is unclear whether fluoride acts by mimicking the γ-phosphate in those complexes.
Previous reports have described an aluminum-independent effect of fluoride on G protein activation (20, 21). In those studies, it was suggested that a GDP-Mg2F3 molecule that is structurally analogous to the GDP-AlF4 molecule is formed and that Mg2F3 is capable of mimicking the γ-phosphate. Although our results support a molecular mechanism for Mg2F3 similar to that previously described for the aluminum-independent activation of Gα proteins, the absence of a requirement for guanine nucleotide in the fluoride-promoted Rho–p190 complex does not support a mechanism that involves the postulated GDP-Mg2F3 molecule. Significantly, it has been reported (16) that fluoride can activate the Gs protein in the absence of GDP, and these investigators also concluded that their results do not support the γ-phosphate-mimicking hypothesis of AlF4 activation. Moreover, fluoride can activate NADPH oxidase in a cell-free system through an aluminum-independent mechanism that appears to involve interaction of the Rac GTPase with an unidentified RacGAP, suggesting a role for fluoride similar to that described herein (34).
Thus, with these previous observations, the results described herein suggest that fluoride can play a distinct role in stabilizing the GTPase transition state that is not likely to be unique to the RhoA–p190 complex. The fact that the p190 RhoGAP catalytic domain has a primary structure that is not obviously distinct from other reported RhoGAPs (10) suggests that the mechanism by which it interacts with its GTPase targets is not atypical. Although structural data clearly support the ability of aluminum fluoride to mimic the γ-phosphate for some GDP-bound GTPases (27, 30, 35), our results strongly indicate that there is another mechanism by which fluoride can interact with GTPases and that the consequences of this interaction appear to be similar to those described for aluminum fluoride. To fully understand such a mechanism, it will most likely be necessary to determine the crystal structure of a GTPase–GAP complex such as that formed between nucleotide-free RhoA and p190 in the presence of fluoride ions.
Acknowledgments
We are grateful to Jim Casanova, Rosemary Foster, and Brenda Schulman for critical reading of the manuscript. S.V. is supported by a fellowship from the Lady Tata Memorial Trust. This work was supported by an award to J.S. from the National Institutes of Health.
ABBREVIATIONS
- GAP
GTPase-activating protein
- GST
glutathione S-transferase
- RGS
regulators of G protein signaling
References
- 1.Wittinghofer A. Curr Biol. 1997;7:R682–R685. doi: 10.1016/s0960-9822(06)00355-1. [DOI] [PubMed] [Google Scholar]
- 2.Bigay J, Deterre P, Pfister C, Chabre M. FEBS Lett. 1985;191:181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 3.Kahn R A. J Biol Chem. 1991;266:15595–15597. [PubMed] [Google Scholar]
- 4.Mittal R, Ahmadian M R, Goody R S, Wittinghofer A. Science. 1996;273:115–117. doi: 10.1126/science.273.5271.115. [DOI] [PubMed] [Google Scholar]
- 5.Ahmadian M R, Mittal R, Hall A, Wittinghofer A. FEBS Lett. 1997;408:315–318. doi: 10.1016/s0014-5793(97)00422-5. [DOI] [PubMed] [Google Scholar]
- 6.Bourne H R, Sanders D A, McCormick F. Nature (London) 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 7.Settleman J, Foster R. Methods Enzymol. 1995;256:105–113. doi: 10.1016/0076-6879(95)56015-7. [DOI] [PubMed] [Google Scholar]
- 8.Hu K Q, Settleman J. EMBO J. 1997;16:473–483. doi: 10.1093/emboj/16.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent S, Settleman J. Mol Cell Biol. 1997;17:2247–2256. doi: 10.1128/mcb.17.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Settleman J, Albright C F, Foster L C, Weinberg R A. Nature (London) 1992;359:153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- 11.Hart M J, Sharma S, elMasry N, Qiu R G, McCabe P, Polakis P, Bollag G. J Biol Chem. 1996;271:25452–25458. doi: 10.1074/jbc.271.41.25452. [DOI] [PubMed] [Google Scholar]
- 12.Ridley A J, Self A J, Kasmi F, Paterson H F, Hall A, Marshall C J, Ellis C. EMBO J. 1993;12:5151–5160. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burbelo P D, Miyamoto S, Utani A, Brill S, Yamada K M, Hall A, Yamada Y. J Biol Chem. 1995;270:30919–30926. doi: 10.1074/jbc.270.52.30919. [DOI] [PubMed] [Google Scholar]
- 14.Foster R, Hu K-Q, Shaywitz D A, Settleman J. Mol Cell Biol. 1994;14:7173–7181. doi: 10.1128/mcb.14.11.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sternweis P C, Gilman A G. Proc Natl Acad Sci USA. 1982;79:4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatani A, Brown A M. J Biol Chem. 1991;266:22872–22877. [PubMed] [Google Scholar]
- 17.Kawase T, Van Breemen C. Eur J Pharmacol. 1992;214:39–44. doi: 10.1016/0014-2999(92)90093-j. [DOI] [PubMed] [Google Scholar]
- 18.Loweth A C, Williams G T, Scarpello J H, Morgan N G. Exp Cell Res. 1996;229:69–76. doi: 10.1006/excr.1996.0344. [DOI] [PubMed] [Google Scholar]
- 19.Ries J, Stein J, Traynor-Kaplan A E, Barrett K E. Am J Physiol. 1997;272:C794–C803. doi: 10.1152/ajpcell.1997.272.3.C794. [DOI] [PubMed] [Google Scholar]
- 20.Antonny B, Bigay J, Chabre M. FEBS Lett. 1990;268:277–280. doi: 10.1016/0014-5793(90)81027-l. [DOI] [PubMed] [Google Scholar]
- 21.Antonny B, Sukumar M, Bigay J, Chabre M, Higashijima T. J Biol Chem. 1993;268:2393–2402. [PubMed] [Google Scholar]
- 22.Hall A, Self A J. J Biol Chem. 1986;261:10963–10965. [PubMed] [Google Scholar]
- 23.Self A J, Hall A. Methods Enzymol. 1995;256:67–76. doi: 10.1016/0076-6879(95)56010-6. [DOI] [PubMed] [Google Scholar]
- 24.Morii N, Kumagai N, Nur E K M S, Narumiya S, Maruta H. J Biol Chem. 1993;268:27160–27163. [PubMed] [Google Scholar]
- 25.Shibata H, Mukai H, Inagaki Y, Homma Y, Kimura K, Kaibushi K, Narumiya S, Ono Y. FEBS Lett. 1996;385:221–224. doi: 10.1016/0014-5793(96)00385-7. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe G, Saito Y, Madaule P, Ischizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka S, Narumiya S. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- 27.Scheffzek K, Ahmadian M R, Kabsch W, Wiesmüller L, Lautwein A, Schmitz F, Wittinghofer A. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 28.Natochin M, Lipkin V M, Artemyev N O. FEBS Lett. 1997;411:179–182. doi: 10.1016/s0014-5793(97)00687-x. [DOI] [PubMed] [Google Scholar]
- 29.Popov S, Yu K, Kozasa T, Wilkie T M. Proc Natl Acad Sci USA. 1997;94:7216–7220. doi: 10.1073/pnas.94.14.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesmer J J, Berman D M, Gilman A G, Sprang S R. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 31.Berman D M, Kozasa T, Gilman A G. J Biol Chem. 1996;271:27209–27212. doi: 10.1074/jbc.271.44.27209. [DOI] [PubMed] [Google Scholar]
- 32.Watson N, Linder M E, Druey K M, Kehrl J H, Blumer K J. Nature (London) 1996;383:172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- 33.Chen C K, Wieland T, Simon M I. Proc Natl Acad Sci USA. 1996;93:12885–12889. doi: 10.1073/pnas.93.23.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfl J, Dagher M C, Fuchs A, Geiszt M, Ligeti E. Eur J Biochem. 1996;239:369–375. doi: 10.1111/j.1432-1033.1996.0369u.x. [DOI] [PubMed] [Google Scholar]
- 35.Sondek J, Lambright D G, Noel J P, Hamm H E, Sigler P B. Nature (London) 1994;372:276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar]