Abstract
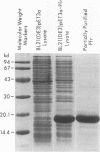
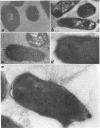
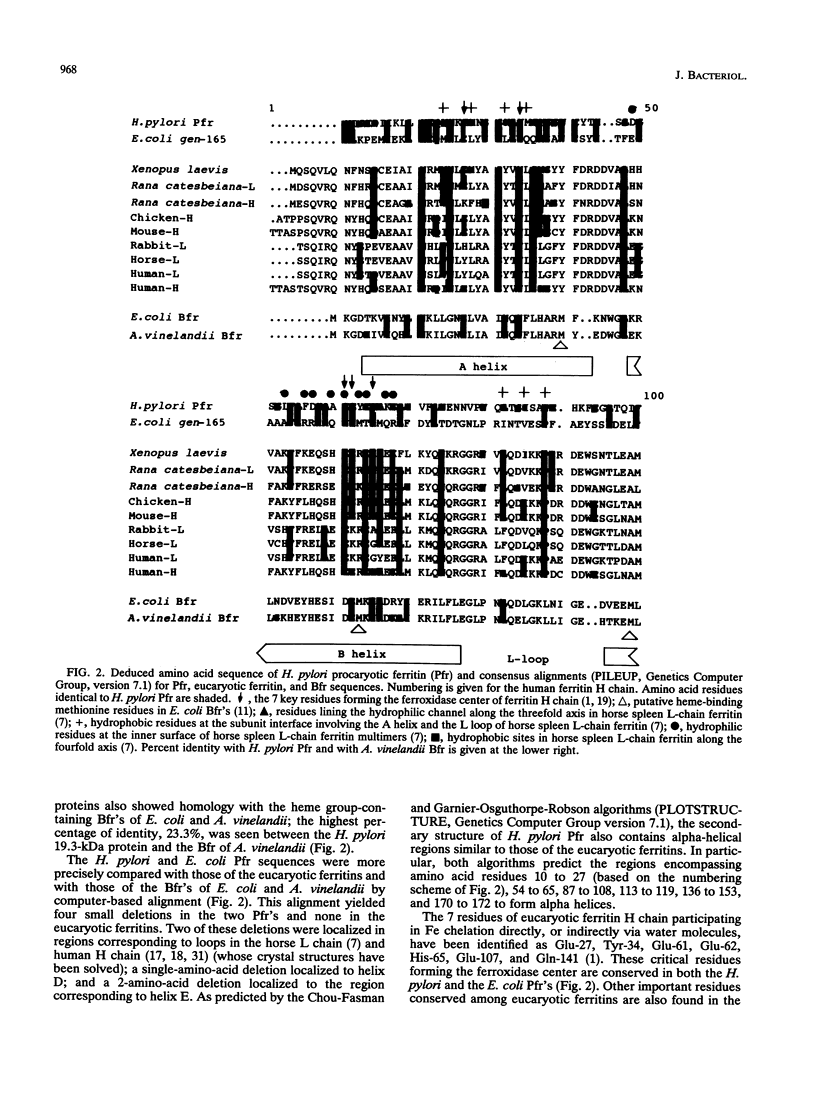
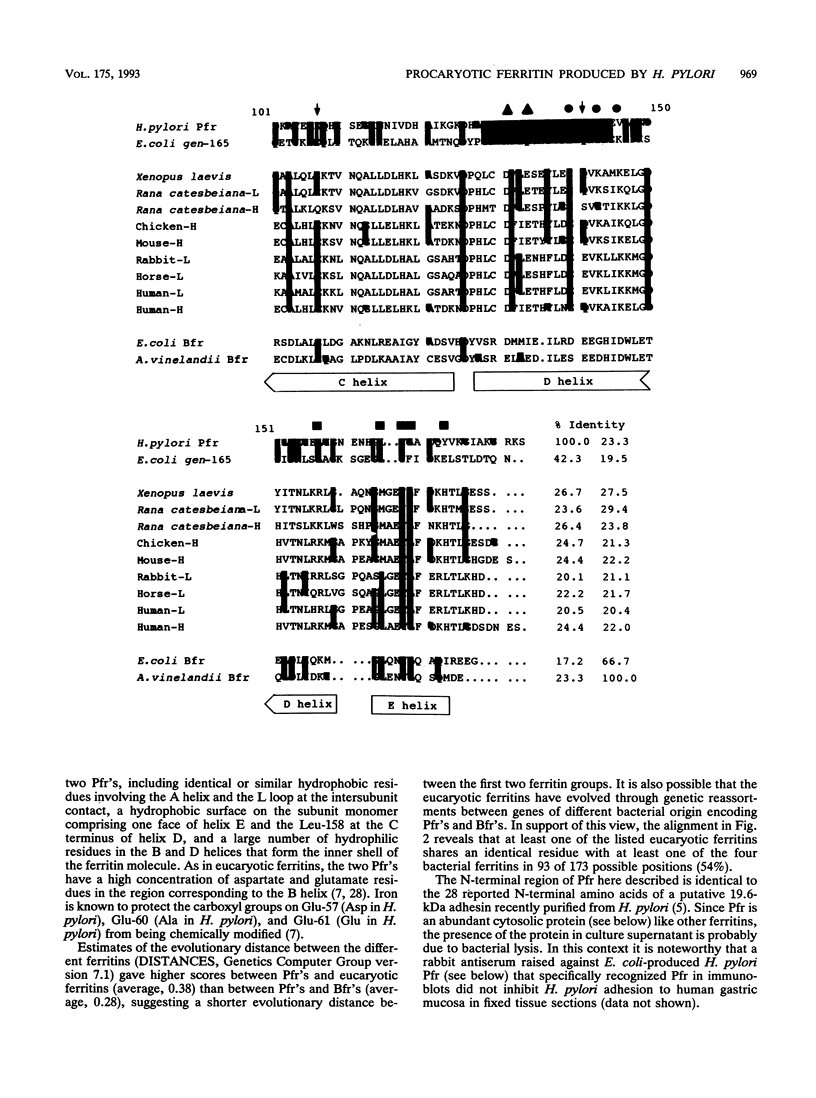
An abundant 19.3-kDa Helicobacter pylori protein has been cloned, and the sequence is homologous with a ferritin-like protein produced by Escherichia coli K-12. Homologies are also present with a number of eucaryotic ferritins, as well as with the heme group-containing bacterioferritins. All amino acids involved in chelation of inorganic iron by ferritins from humans and other higher species are conserved in the H. pylori protein. Consistent with the structural data indicating an iron-binding function, E. coli overexpressing the H. pylori ferritin-like protein accumulates almost 10 times more nonheme iron than vector controls, and the iron-binding activity copurifies with the 19.3-kDa protein. Immunoelectron microscopy of H. pylori, as well as of E. coli overexpressing the H. pylori gene, demonstrates that the gene product has a cytoplasmic location where it forms paracrystalline inclusions. On the basis of these structural and functional data, we propose that the H. pylori gene product (termed Pfr) forms the basis for a second class of bacterial ferritins designed to store nonheme iron.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Smith J. M., Yewdall S. J., Guest J. R., Harrison P. M. Bacterioferritins and ferritins are distantly related in evolution. Conservation of ferroxidase-centre residues. FEBS Lett. 1991 Nov 18;293(1-2):164–168. doi: 10.1016/0014-5793(91)81177-a. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem. 1987 May 4;164(3):485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Austin J. W., Kostrzynska M., Trust T. J. Production of a conserved adhesin by the human gastroduodenal pathogen Helicobacter pylori. J Bacteriol. 1992 Apr;174(8):2539–2547. doi: 10.1128/jb.174.8.2539-2547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach F. A., Anderegg J. W. An x-ray scattering study of ferritin and apoferritin. J Mol Biol. 1965 Dec;14(2):458–473. doi: 10.1016/s0022-2836(65)80196-6. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S. Duodenal ulcer, Campylobacter pylori, and the "leaking roof" concept. Lancet. 1988 Dec 24;2(8626-8627):1467–1469. doi: 10.1016/s0140-6736(88)90942-7. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Grossman M. J., Hinton S. M., Minak-Bernero V., Slaughter C., Stiefel E. I. Unification of the ferritin family of proteins. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2419–2423. doi: 10.1073/pnas.89.6.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuhara M., Takamune K., Takata R. Cloning and sequencing of an Escherichia coli K12 gene which encodes a polypeptide having similarity to the human ferritin H subunit. Mol Gen Genet. 1991 Mar;225(3):510–513. doi: 10.1007/BF00261694. [DOI] [PubMed] [Google Scholar]
- Lawson D. M., Artymiuk P. J., Yewdall S. J., Smith J. M., Livingstone J. C., Treffry A., Luzzago A., Levi S., Arosio P., Cesareni G. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature. 1991 Feb 7;349(6309):541–544. doi: 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. M., Treffry A., Artymiuk P. J., Harrison P. M., Yewdall S. J., Luzzago A., Cesareni G., Levi S., Arosio P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989 Aug 28;254(1-2):207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- Levi S., Luzzago A., Cesareni G., Cozzi A., Franceschinelli F., Albertini A., Arosio P. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem. 1988 Dec 5;263(34):18086–18092. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Moore G. R., Mann S., Bannister J. V. Isolation and properties of the complex nonheme-iron-containing cytochrome b557 (bacterioferritin) from Pseudomonas aeruginosa. J Inorg Biochem. 1986 Oct-Nov;28(2-3):329–336. doi: 10.1016/0162-0134(86)80097-6. [DOI] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Xu S., Chakraborty P. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages. J Cell Sci. 1992 Dec;103(Pt 4):1193–1210. doi: 10.1242/jcs.103.4.1193. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Wyatt J. I., Dixon M. F. Chronic gastritis--a pathogenetic approach. J Pathol. 1988 Feb;154(2):113–124. doi: 10.1002/path.1711540203. [DOI] [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Sperling R., Bauminger E. R., Cohen S. G., Ofer S. The composition and the structure of bacterioferritin of Escherichia coli. Biochem J. 1981 Jul 1;197(1):171–175. doi: 10.1042/bj1970171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdall S. J., Lawson D. M., Artymiuk P. J., Treffry A., Harrison P. M., Luzzago A., Cesareni G., Levi S., Arosio P. Structural studies on recombinant human ferritins. Biochem Soc Trans. 1990 Oct;18(5):1028–1029. doi: 10.1042/bst0181028. [DOI] [PubMed] [Google Scholar]