Abstract
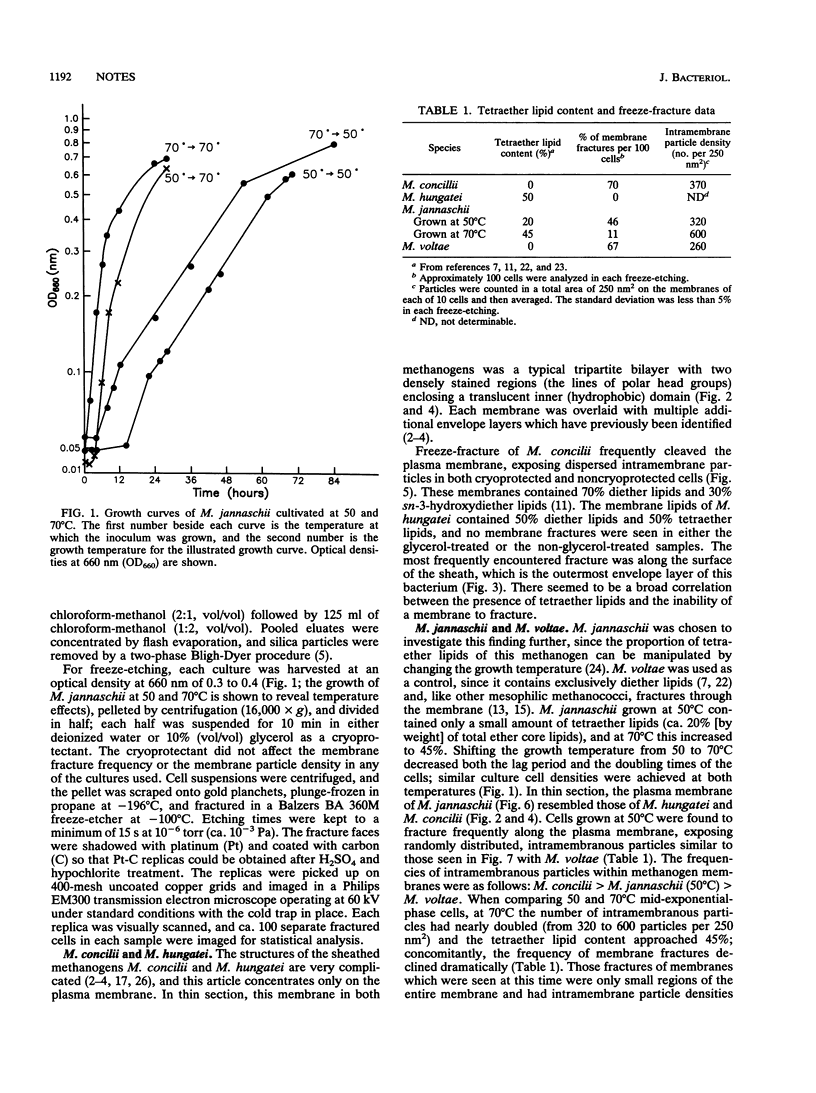
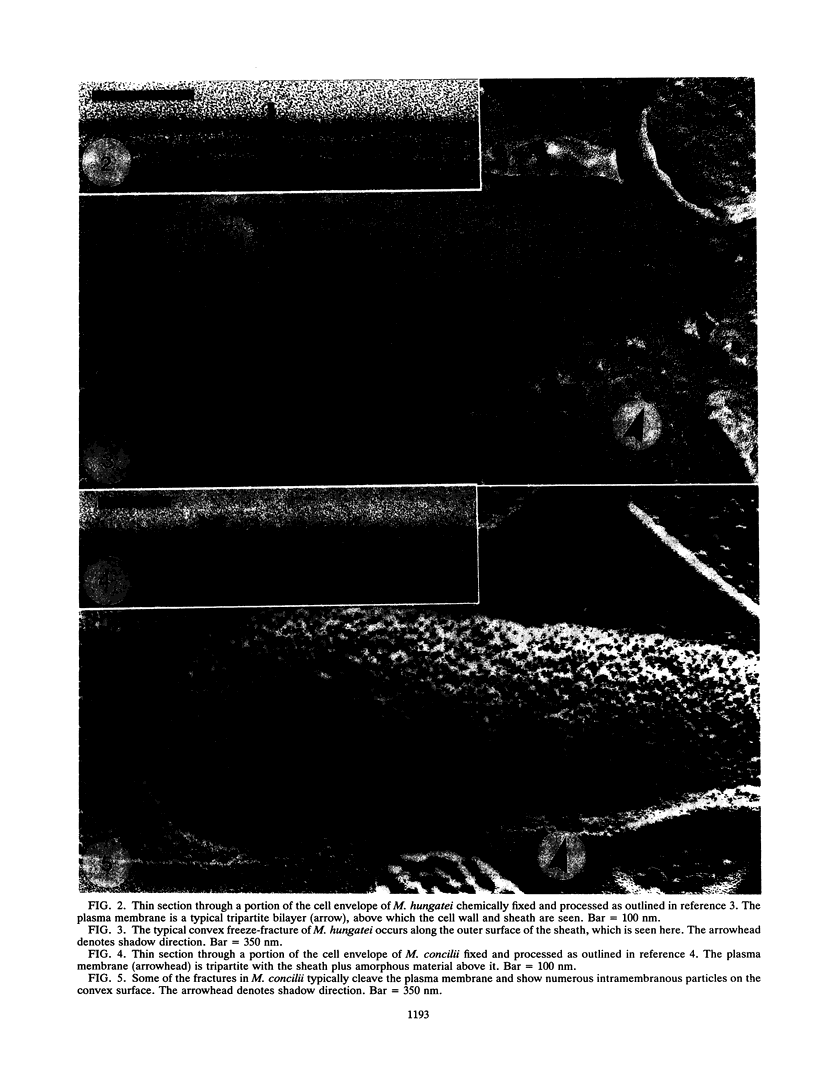
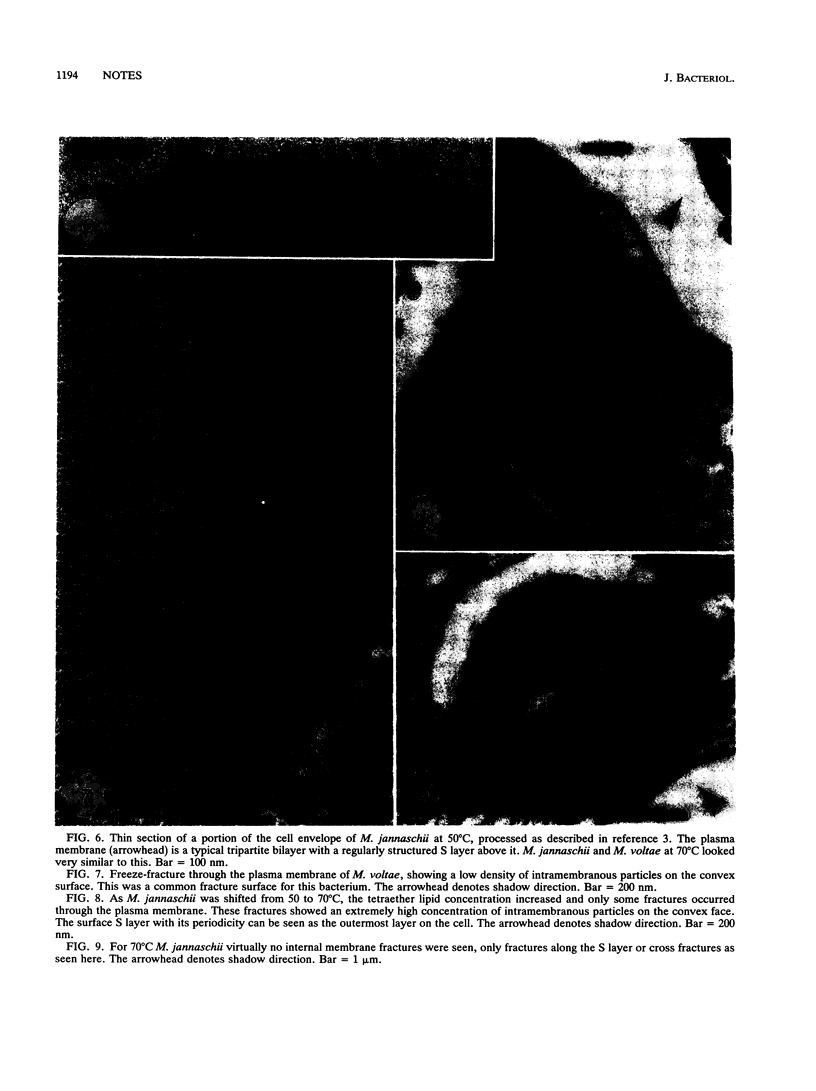
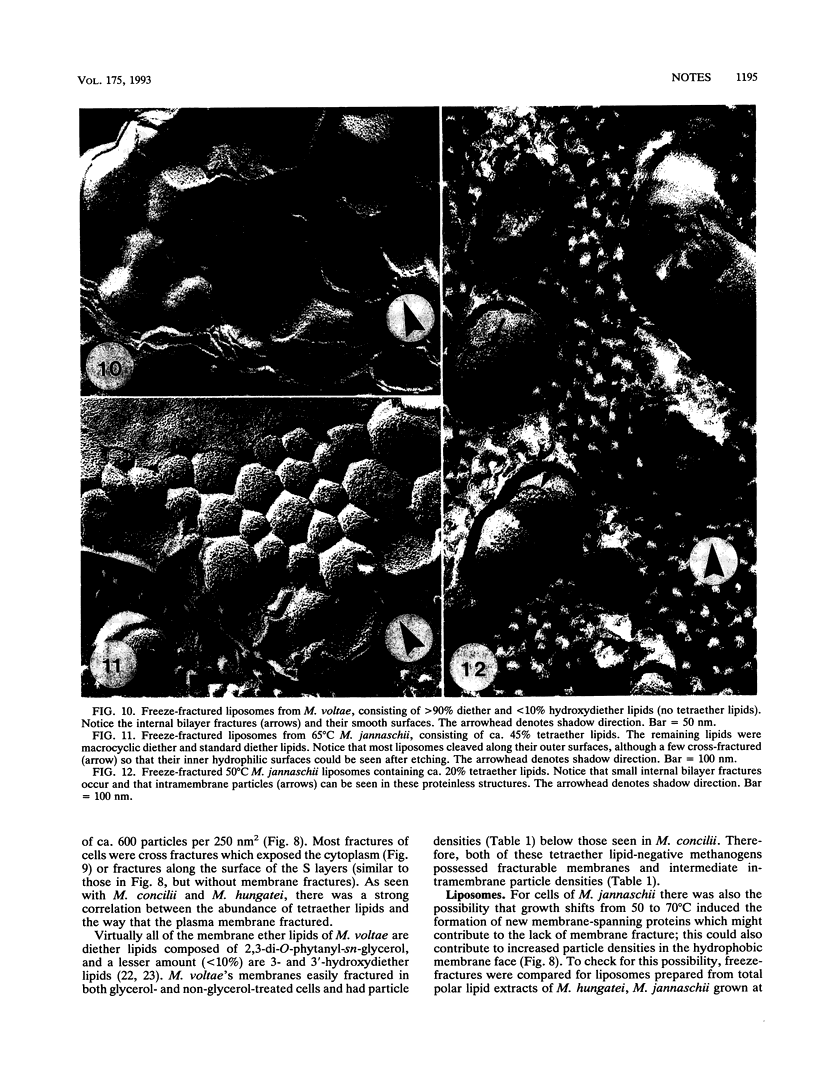
Methanospirillum hungatei GP1 contained 50% of its ether core lipids (polar lipids less head groups) as tetraether lipids, and its plasma membrane failed to fracture along its hydrophobic domain during freeze-etching. The membrane of Methanosaeta ("Methanothrix") concilii did not contain tetraether lipids and easily fractured to reveal typical intramembranous particles. Methanococcus jannaschii grown at 50 degrees C contained 20% tetraether core lipids, which increased to 45% when cells were grown at 70 degrees C. The frequency of membrane fracture was reduced as the membrane-spanning tetraether lipids approached 45%. As the tetraether lipid content increased, and while fracture was still possible, the particle density in the membrane increased; these added particles could be tetraether lipid complexes torn from the opposing membrane face. The diether membrane (no tetraether lipid) of Methanococcus voltae easily fractured, and the intramembranous particle density was low. Protein-free liposomes containing tetraether core lipids (ca. 45%) also did not fracture, whereas those made up exclusively of diether lipids did split, indicating that tetraether lipids add considerable vertical stability to the membrane. At tetraether lipid concentrations below 45%, liposome bilayers fractured to reveal small intramembranous particles which we interpret to be tetraether lipid complexes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Breuil C., Patel G. B. Composition of Methanospirillum hungatii GP1 during growth on different media. Can J Microbiol. 1980 May;26(5):577–582. doi: 10.1139/m80-102. [DOI] [PubMed] [Google Scholar]
- Choquet C. G., Patel G. B., Beveridge T. J., Sprott G. D. Formation of unilamellar liposomes from total polar lipid extracts of methanogens. Appl Environ Microbiol. 1992 Sep;58(9):2894–2900. doi: 10.1128/aem.58.9.2894-2900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comita P. B., Gagosian R. B., Pang H., Costello C. E. Structural elucidation of a unique macrocyclic membrane lipid from a new, extremely thermophilic, deep-sea hydrothermal vent archaebacterium, Methanococcus jannaschii. J Biol Chem. 1984 Dec 25;259(24):15234–15241. [PubMed] [Google Scholar]
- De Rosa M., Gambacorta A., Gliozzi A. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol Rev. 1986 Mar;50(1):70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante G., Brisson J. R., Patel G. B., Ekiel I., Sprott G. D. Structures of minor ether lipids isolated from the aceticlastic methanogen, Methanothrix concilii GP6. J Lipid Res. 1989 Oct;30(10):1601–1609. [PubMed] [Google Scholar]
- Ferrante G., Richards J. C., Sprott G. D. Structures of polar lipids from the thermophilic, deep-sea archaeobacterium Methanococcus jannaschii. Biochem Cell Biol. 1990 Jan;68(1):274–283. doi: 10.1139/o90-038. [DOI] [PubMed] [Google Scholar]
- Jones J. B., Bowers B., Stadtman T. C. Methanococcus vannielii: ultrastructure and sensitivity to detergents and antibiotics. J Bacteriol. 1977 Jun;130(3):1357–1363. doi: 10.1128/jb.130.3.1357-1363.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval S. F., Jarrell K. F. Ultrastructure and biochemistry of the cell wall of Methanococcus voltae. J Bacteriol. 1987 Mar;169(3):1298–1306. doi: 10.1128/jb.169.3.1298-1306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. B. A contrary view of the proposal to assign a neotype strain for Methanothrix soehngenii. Int J Syst Bacteriol. 1992 Apr;42(2):324–326. doi: 10.1099/00207713-42-2-324. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Ekiel I., Dicaire C. Novel, acid-labile, hydroxydiether lipid cores in methanogenic bacteria. J Biol Chem. 1990 Aug 15;265(23):13735–13740. [PubMed] [Google Scholar]
- Sprott G. D., Meloche M., Richards J. C. Proportions of diether, macrocyclic diether, and tetraether lipids in Methanococcus jannaschii grown at different temperatures. J Bacteriol. 1991 Jun;173(12):3907–3910. doi: 10.1128/jb.173.12.3907-3910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D. Structures of archaebacterial membrane lipids. J Bioenerg Biomembr. 1992 Dec;24(6):555–566. doi: 10.1007/BF00762348. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Bowen V. G. Fine structure of Methanospirillum hungatii. J Bacteriol. 1975 Jan;121(1):373–380. doi: 10.1128/jb.121.1.373-380.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]





