Abstract
The mRNA for organic anion transport protein (oatp) was previously shown to be present in abundance in liver and kidney, and in small amounts in brain. Data obtained from experiments with reverse transcriptase–PCR techniques and in situ hybridization analysis showed that the oatp mRNA is present within the brain, localized to the choroid plexus. A sequence-specific antibody to the oatp polypeptide demonstrated the presence of the expected polypeptide with a molecular weight of 80,000 plus an immunoreactive species with a higher molecular weight in preparations of choroid plexus membranes. Examination of the choroid plexus by fluorescence confocal microscopy revealed that immunoreactive oatp polypeptide is localized to the apical surface of the choroid plexus epithelial cells, which contacts the cerebrospinal fluid. This localization of oatp is consistent with previous experiments showing vectorial transport of organic anions between the choroid plexus and the cerebrospinal fluid.
Keywords: bilirubin, oatp
Transport of organic anions as a mechanism for clearance from the cerebrospinal fluid was convincingly demonstrated by Pappenheimer and coworkers (1). It was suggested that the choroid plexus could be the site of this transport, drawing support from earlier observations that choroid plexus epithelium in culture forms sacs in which these compounds could be concentrated (2). Detailed kinetic studies performed in isolated choroid plexus tissue have confirmed and characterized this organic anion transport process as similar to those in liver and kidney (3, 4). A cDNA was recently expression-cloned from rat liver on the basis of its conferring upon cells the ability to transport organic anions such as sulfobromophthalein (5). This cDNA encodes a protein present in liver and kidney that has been termed organic anion transport protein (oatp).
oatp cDNA encodes a protein with a calculated molecular weight of 74,034 and 12 predicted transmembrane domains (5). Sequence-specific antibodies have been used to localize the oatp protein to the basolateral surface of hepatocytes, and the apical portion of the S3 segment of the proximal tubule of the rat kidney (6). The amino acid sequence of this transporter is structurally unrelated to other previously studied transporters. However, a protein termed matrin F/G, a DNA-binding zinc-finger protein, was found to be 38% identical to oatp in searches of protein sequence data bases (7). Schuster and Wolkoff were able to show that this cDNA actually encoded a prostaglandin transporter, 99 aa longer than that originally predicted (8). It is likely that oatp may be a member of a new family of integral membrane transporters, with more members yet to be identified.
Previous studies in which Northern blot analysis was performed at high stringency showed that tissues other than liver contained mRNA that hybridized to the oatp cDNA clone (5). Although total RNA isolated from whole brain was positive for oatp mRNA, an approximately 10-fold longer exposure time was required to reveal a signal of intensity similar to that seen in total RNA prepared from rat liver. Within the brain, the choroid plexus epithelium is the blood–cerebrospinal fluid barrier and is known to share many functional properties with hepatocytes (9). For example, the transporters for glucose (10), amino acids (11), vitamins (12), nucleosides (13), and fatty acids (14) are present. Organic anion transport has also been demonstrated both in isolated preparations of choroid plexus and in vivo (4, 15). Specific binding of radiolabeled bilirubin to the choroid plexus has also been described (16). In this study, we demonstrate that, within the brain, oatp mRNA and protein are localized to the choroid plexus.
MATERIALS AND METHODS
Immunoblot Analysis of oatp.
An antiserum was raised in rabbits to a synthetic peptide corresponding to a region near the carboxyl terminus of oatp, as described (6). Sprague–Dawley rats were used in all experiments. Freshly extirpated tissue was homogenized in the presence of protease inhibitors, and the membrane pellet was extracted with 0.1 M sodium carbonate to remove loosely associated proteins, as described (17). The sodium carbonate-insoluble pellets were dissolved in sample buffer, and the proteins were separated by SDS/PAGE (10% gel) (18). Proteins were transferred to poly(vinylidene difluoride) membranes (Millipore) with a dryblotting apparatus (Hoefer). After blocking with nonfat milk solution, the membranes were incubated with antibody for 4 h before developing for luminescent detection (Amersham).
Fluorescent Confocal Microscopy.
oatp and actin in choroid plexus were immunolocalized as described (19). Briefly, freshly extirpated choroid plexus was fixed by immersion in 4% paraformaldehyde for 3 h at 4°C. After blocking in sodium borohydride and incubation in goat serum, BSA, and Tween detergent, the whole choroid plexus tissue was sequentially exposed to a sequence-specific anti-oatp rabbit serum (6) (dilution 1:100). Then the whole tissue was exposed to goat anti-rabbit IgG labeled with Cy-3 (dilution 1:100; Jackson ImmunoResearch) and then to the fluorescein isothiocyanate derivative of phalloidin (Molecular Probes). The tissue was examined with an inverted Nikon fluorescence microscope attached to the Bio-Rad Mrc 600 confocal laser imaging system equipped with a krypton/argon laser. Single optical section and x and z axes analyses were performed for the entire depth of the tissue, approximately 20 μm.
Reverse Transcriptase (RT)–PCR Detection of oatp mRNA.
Total RNA of choroid plexus and liver was prepared with TRIzol reagent (GIBCO/BRL) by a modification of the guanidine thiocyanate procedure (20). RT-PCR was used to detect the presence of oatp mRNA. The 22-bp 5′ primer corresponded to nucleotides 1925–1946 of the cDNA sequence 5′-TCTGCCTGCCTTCTTCATTCTG (GenBank accession no. L19031L19031). The 24-bp 3′ primer corresponded to nucleotides 2349–2326 of the mRNA sequence 5′-GTAGTGTGGGGGTCACCATAAAAG. The expected product of these primers is 425 bp. Enzymes and the thermal cycler were from Perkin–Elmer.
In Situ Hybridization.
In situ hybridization was performed as described (21). Briefly, parasagittal crysostat sections (14 μm) from adult rat brain were hybridized for 18 h at 43°C with 60-mer oligonucleotides, complementary to positions 2031–2090 of the rat oatp cDNA, end-labeled with [35S]dATP (New England Nuclear). The hybridization solution contained 50% deionized formamide, 40% dextran sulfate filtrate, 0.25 mg/ml tRNA, 0.25 mg/ml polyadenylic acid, 0.25 mg/ml denatured herring sperm DNA, and labeled oligonucleotide probe (300,000 cpm/50 μl). Sections were rinsed with a solution containing 0.2 M NaCl and 20 mM sodium citrate (1× SSC) and twice with 0.4× SSC at 55°C. Sections were then dehydrated and covered with β-max Hyperfilm (Amersham) for 4–21 days. In control experiments, the hybridization was performed in the presence of a 10-fold excess of unlabeled oligonucleotide probe.
RESULTS
RT-PCR Analysis of oatp mRNA in Choroid Plexus.
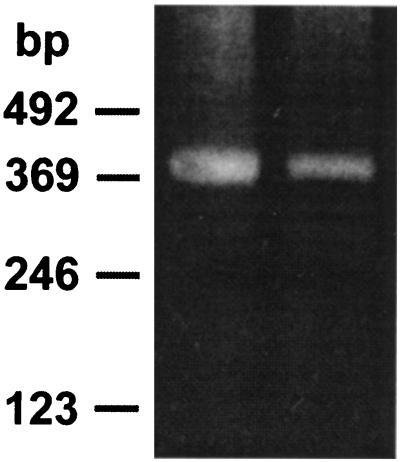
Because the choroid plexus is a single layer of cells, forming only a small proportion of the volume of the brain in the adult, RT-PCR was used in the initial analysis for the presence of oatp mRNA. Primers were selected from the 3′ region of the mRNA and were expected to yield a PCR product of 425 bp. The positive control RNA prepared from rat liver yielded a product of this size, and RNA from choroid plexus produced a band of 425 bp as well (Fig. 1).
Figure 1.
RT-PCR analysis of oatp mRNA in choroid plexus. The reaction products separated by agarose gel electrophoresis are shown for mRNA prepared from liver (left lane) and choroid plexus (right lane). The molecular weight markers are shown on the left.
In Situ Hybridization Analysis of oatp mRNA in Choroid Plexus.
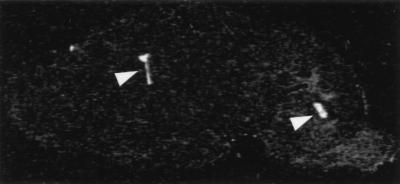
To further confirm the presence of the oatp transcript in choroid plexus and to determine whether this localization is unique, in situ hybridization analysis was performed. An example in a sagittal section of rat brain is shown in Fig. 2. The choroid plexus of the lateral and fourth ventricles is intensely labeled, with no labeling detected in other parts of the brain. Examination of the set of sagittal sections showed specific labeling of the choroid plexus in the lateral, third, and fourth ventricles. No specific labeling was detected in other regions of the brain, even with longer exposure times.
Figure 2.
In situ hybridization analysis of oatp mRNA in rat brain. A sagittal section of the brain is shown after hybridization with a radiolabeled, oatp-specific oligonucleotide probe and exposure to film. Arrowheads point out the intense labeling in the lateral (left arrowhead) and fourth (right arrowhead) ventricles.
Immunological Detection of oatp Polypeptide.
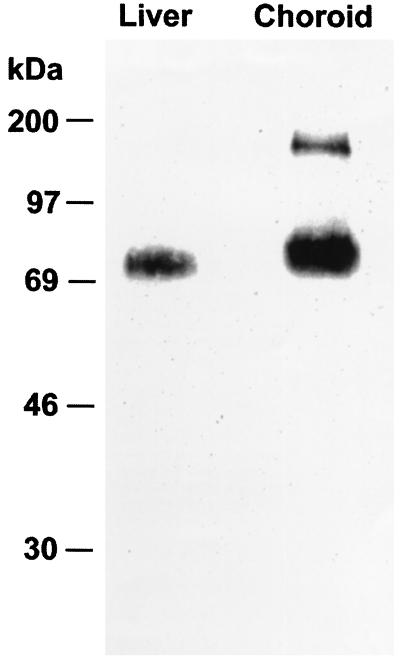
The oatp polypeptide cannot be detected in immunoblots of whole tissue homogenates (6). It is necessary to prepare membrane fractions enriched in integral membrane proteins by extraction with sodium carbonate to visualize the oatp antigen. As seen in Fig. 3, this method of analysis readily revealed the presence of a single immunoreactive polypeptide with a molecular weight of 80,000 in rat liver, as noted in previous studies (6). Samples from choroid plexus showed an immunoreactive band at a molecular weight of 80,000 and a band at a molecular weight of 160,000.
Figure 3.
Immunoblot analysis of the oatp polypeptide. The immunoreactive bands in liver (left lane) and choroid plexus (right lane) are shown. Molecular weight markers are at the left.
Colocalization of oatp and Actin.
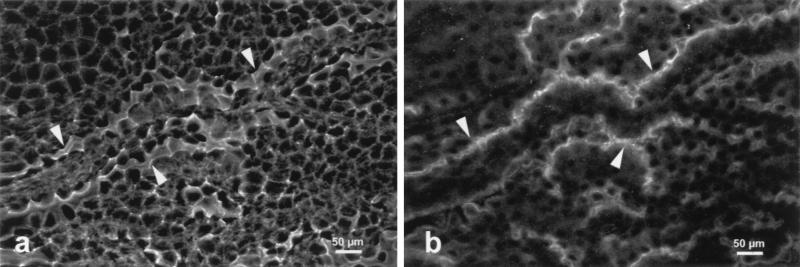
Because the choroid plexus is a polarized tissue with distinct functional faces, confocal microscopy was performed to determine the subcellular localization of oatp. The tissue was examined whole, and thus appeared folded on the surface of the slide. The basal surface of these cells is apposed to a capillary bed, whereas the apical surface, facing the cerebrospinal fluid, was elaborated with microvilli. Fig. 4 illustrates the confocal analysis of the colocalization of oatp and actin. In Fig. 4a, actin filaments revealed by the fluorescein isothiocyanate derivative of phalloidin are evident around the entire subplasma membrane surface of the choroid plexus epithelium. At the apical surface, the actin localization appears as a wide band of fluorescence where the microvilli are located, and where the apical surface is sectioned tangentially. In Fig. 4b, which is the same optical section as that in Fig. 4a, the oatp detected by a Cy-3-labeled secondary antibody is present only at the surface facing the cerebrospinal fluid, that is, the apical surface.
Figure 4.
Confocal microscopy of choroid plexus. Freshly extirpated choroid plexus was incubated either with the fluorescein isothiocyanate derivative of phalloidin to reveal actin (a) or antiserum to oatp followed by a rhodamine-labeled secondary antibody (b). Arrowheads point out the intense staining seen at the apical surface of the epithelial cells.
DISCUSSION
The liver efficiently removes organic anions, such as sulfobromophthalein and bilirubin, from the circulation, with a single-pass extraction fraction as high as 50% (22). The efficiency of this process is important, as these organic anions are potentially toxic. Similar transport processes have been described in the choroid plexus, which shares other physiological transport processes with both liver and kidney (9). Choroid transport may represent a major factor for permeation of these, or related, compounds into or out of the cerebrospinal fluid.
In this report, RT-PCR experiments demonstrated that the mRNA for oatp is present in choroid plexus. This result is consistent with the previously published Northern blot analysis results for whole brain total RNA, which demonstrated major mRNA species of 2.8 and 4.0 kb (5). The longer exposure required for the brain RNA samples indicated that either the levels of the mRNA are lower throughout the brain or it is localized to a limited brain region. These studies were confirmed by in situ hybridization experiments, which revealed a very strong signal localized to the choroid plexus in all ventricles, with only background signal in the remainder of the brain.
The oatp polypeptide can be detected by a sequence-specific antibody to the carboxyl-terminal peptide of the predicted amino acid sequence (6). Although a major peptide (Mr = 80,000) was detected in samples from the choroid plexus and liver; a much larger polypeptide (Mr near 160,000) was also found only in the choroid plexus membranes. The samples had been reduced with dithiothreitol before analysis, so the larger polypeptide is unlikely to represent a dimer. Furthermore, the liver sample was prepared at the same time and did not show this high molecular weight species. It is possible that the difference in apparent molecular weights is due to posttranslational modification specific to the choroid plexus. The types and extent of posttranslational modifications in both liver and choroid plexus are subjects for future investigation.
Localization of the oatp polypeptide within the choroid plexus is of particular interest, because of previous physiological experiments indicating vectorial transport to and from the cerebrospinal fluid (9). Consistent with this body of work is our identification of the apical surface of the choroid plexus as the site of oatp in this organ. The apical plasma membranes are differentiated, with microvilli on this surface which faces the cerebrospinal fluid. This feature provides a large surface area for transport and is consistent with the observed rapid removal of organic anions from the brain (1, 3, 4). Pappenheimer reported that the choroid plexus of the fourth ventricle, but not the lateral ventricles, was responsible for organic anion transport in the goat brain (1). The present finding of oatp in the lateral ventricles in addition to the fourth ventricle may simply represent a species difference. The location of oatp in the choroid plexus may provide the means by which various organic acids, including pharmaceutical compounds, are kept from reaching high concentrations in the brain.
Acknowledgments
This research was supported by grants from the National Institutes of Health (DK23026, DK41296, and CA06576), a grant from the March of Dimes Foundation, and grants from the Swiss National Science Foundation (31–45536.95 and 31–3632792). Confocal microscopy was performed in the Albert Einstein College of Medicine Analytical Imaging Facility.
Footnotes
Abbreviations: oatp, organic anion transporter protein; RT, reverse transcriptase.
References
- 1.Pappenheimer J R, Heisey S R, Jordan E F. Am J Physiol. 1961;200:1–10. doi: 10.1152/ajplegacy.1961.200.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Cameron G. Anat Rec. 1953;117:115–123. doi: 10.1002/ar.1091170109. [DOI] [PubMed] [Google Scholar]
- 3.Barany E H. Acta Physiol Scand. 1973;88:412–429. doi: 10.1111/j.1748-1716.1973.tb05470.x. [DOI] [PubMed] [Google Scholar]
- 4.Bratlid D. Clin Perinatol. 1990;17:449–465. [PubMed] [Google Scholar]
- 5.Jacquemin E, Hagenbuch B, Stieger B, Wolkoff A W, Meier P J. Proc Natl Acad Sci USA. 1994;91:133–137. doi: 10.1073/pnas.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergwerk A J, Shi X, Ford A C, Kanai N, Jacquemin E, Burk R D, Bai S, Novikoff P M, Stieger B, Meier P J, Schuster V L, Wolkoff A W. Am J Physiol. 1996;271:G231–G238. doi: 10.1152/ajpgi.1996.271.2.G231. [DOI] [PubMed] [Google Scholar]
- 7.Hakes D J, Berezney R. Proc Natl Acad Sci USA. 1991;88:6186–6190. doi: 10.1073/pnas.88.14.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanai N, Lu R, Satriano J A, Bao Y, Wolkoff A W, Schuster V L. Science. 1995;268:866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- 9.Spector R, Johanson C E. Sci Am. 1989;261(5):68–74. doi: 10.1038/scientificamerican1189-68. [DOI] [PubMed] [Google Scholar]
- 10.Harik S I, Kalaria R N, Andersson L, Lundahl P, Perry G. J Neurosci. 1990;10:3862–3872. doi: 10.1523/JNEUROSCI.10-12-03862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoll J, Wadhwani K C, Smith Q R. J Neurochem. 1993;60:1956–1959. doi: 10.1111/j.1471-4159.1993.tb13428.x. [DOI] [PubMed] [Google Scholar]
- 12.Spector R. Neurochem Res. 1987;12:27–31. doi: 10.1007/BF00971360. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Yuan G, Brett C M, Hui A C, Giacomini K M. J Biol Chem. 1992;267:8813–8818. [PubMed] [Google Scholar]
- 14.Kim C S, Roe C R. Brain Res. 1991;554:193–197. doi: 10.1016/0006-8993(91)90188-2. [DOI] [PubMed] [Google Scholar]
- 15.Ohsugi M, Sato H, Yamamura H. Biol Neonate. 1992;62:416–423. doi: 10.1159/000327433. [DOI] [PubMed] [Google Scholar]
- 16.Danbolt C, Hansen T W R, Oyasoeter S, Storm-Mathisen J, Bratlid D. Biol Neonate. 1993;63:35–39. doi: 10.1159/000243905. [DOI] [PubMed] [Google Scholar]
- 17.Fujiki Y, Hubbard A L, Fowler S, Lazarow P B. J Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Novikoff P M, Cammer M, Tao L, Oda H, Wolkoff A W, Satir P. J Cell Sci. 1996;109:21–32. doi: 10.1242/jcs.109.1.21. [DOI] [PubMed] [Google Scholar]
- 20.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 21.Kokaia M, Pratt G D, Elmer E, Bengzon J, Fritschy J-M, Kokaia Z, Lindvall O, Mohler H. Mol Brain Res. 1996;23:323–332. doi: 10.1016/0169-328x(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury J R, Wolkoff A W, Chowdhury N R, Arias I M. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 2161–2208. [Google Scholar]