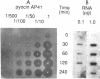
Abstract
Most strains of Pseudomonas aeruginosa produce various types of bacteriocins (pyocins), namely, R-, F-, and S-type pyocins. The production of all types of pyocins was shown to be regulated by positive (prtN) and negative (prtR) regulatory genes. The prtN gene activates the expression of various pyocin genes, probably by the interaction of its product with the DNA sequences conserved in the 5' noncoding regions of the pyocin genes. The prtR gene represses the expression of the prtN gene, and its product, predicted from the nucleotide sequence, has a structure characteristic of phage repressors and seems to be inactivated by the RecA protein activated by DNA damage. A model for the regulation of the pyocin genes is proposed.
Full text
PDF



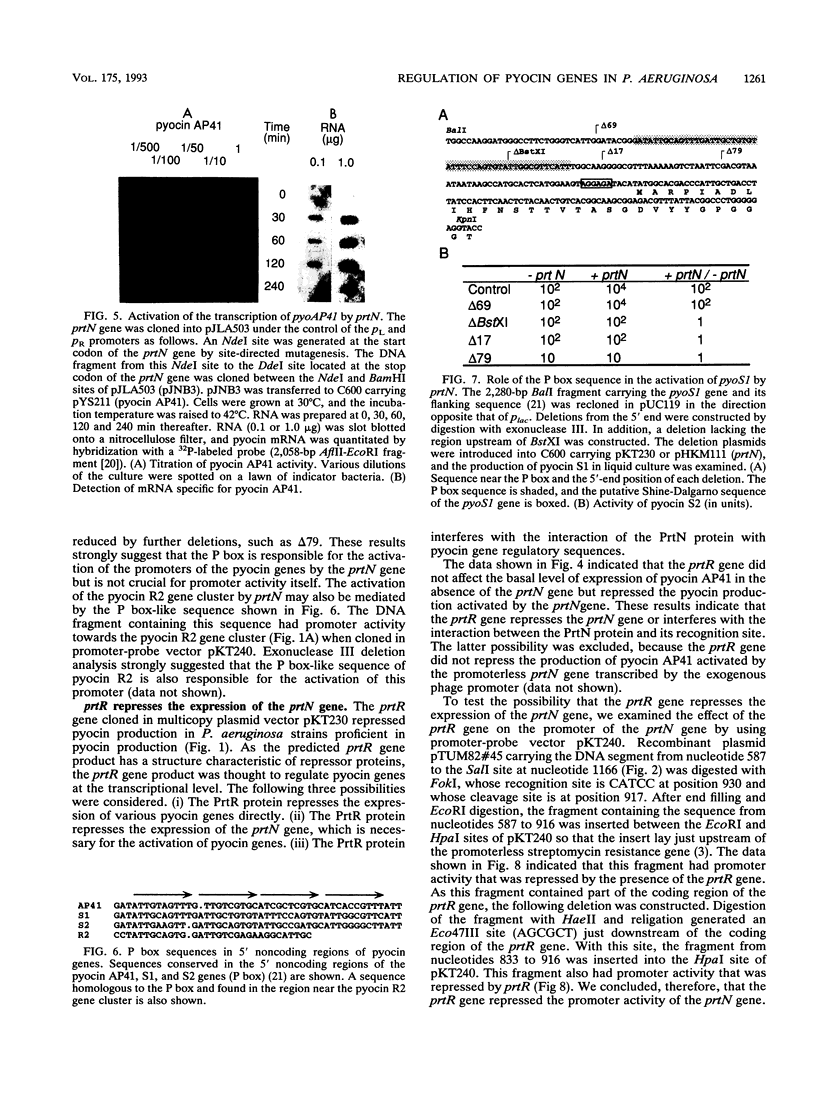
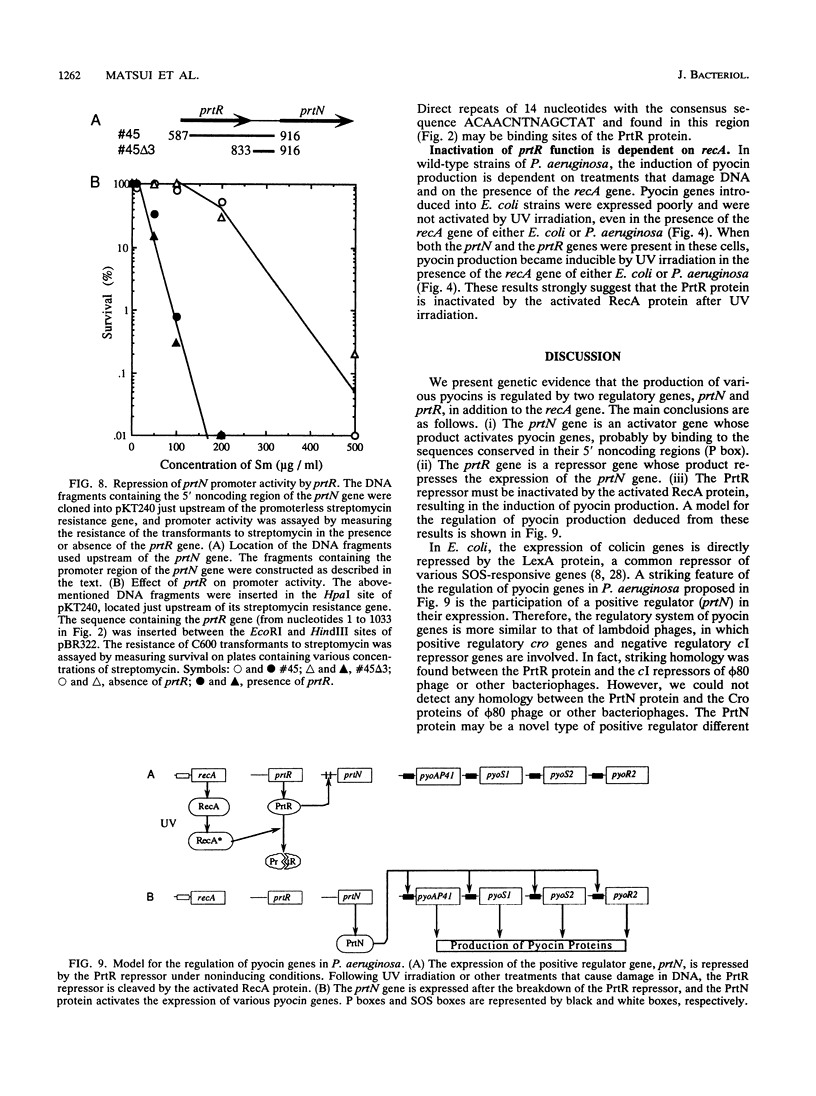

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M. M., Amann E., Lurz R., Rückert B., Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983 Dec;26(2-3):273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Calero S., Garriga X., Barbé J. One-step cloning system for isolation of bacterial lexA-like genes. J Bacteriol. 1991 Nov;173(22):7345–7350. doi: 10.1128/jb.173.22.7345-7350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Kishi F., Nakazawa A. Direct participation of lexA protein in repression of colicin E1 synthesis. J Bacteriol. 1982 Jun;150(3):1479–1481. doi: 10.1128/jb.150.3.1479-1481.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y., Ogawa T., Ogawa H. Cleavage of bacteriophage phi 80 CI repressor by RecA protein. J Mol Biol. 1988 Aug 5;202(3):565–573. doi: 10.1016/0022-2836(88)90286-0. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Nakatani T., Hase T., Matsubara H., Ogawa H. Regulation of SOS functions: purification of E. coli LexA protein and determination of its specific site cleaved by the RecA protein. Cell. 1981 Dec;27(3 Pt 2):515–522. doi: 10.1016/0092-8674(81)90393-7. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Kageyama M. Comparative study of F-type pyocins of Pseudomonas aeruginosa. J Biochem. 1981 Jun;89(6):1721–1736. doi: 10.1093/oxfordjournals.jbchem.a133372. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Kokjohn T. A. Expression of the recA gene of Pseudomonas aeruginosa PAO is inducible by DNA-damaging agents. J Bacteriol. 1988 May;170(5):2385–2387. doi: 10.1128/jb.170.5.2385-2387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y., Kageyama M. Genetic determinant of pyocin AP41 as an insert in the Pseudomonas aeruginosa chromosome. J Bacteriol. 1984 May;158(2):562–570. doi: 10.1128/jb.158.2.562-570.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y., Kageyama M. The sequence and function of the recA gene and its protein in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1987 Jul;208(3):412–419. doi: 10.1007/BF00328132. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Yocum R. R., Doolittle R. F., Lewis M., Pabo C. O. Homology among DNA-binding proteins suggests use of a conserved super-secondary structure. Nature. 1982 Jul 29;298(5873):447–451. doi: 10.1038/298447a0. [DOI] [PubMed] [Google Scholar]
- Schauder B., Blöcker H., Frank R., McCarthy J. E. Inducible expression vectors incorporating the Escherichia coli atpE translational initiation region. Gene. 1987;52(2-3):279–283. doi: 10.1016/0378-1119(87)90054-0. [DOI] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S., Kageyama M. Genetic determinant of pyocin R2 in Pseudomonas aeruginosa PAO. I. Localization of the pyocin R2 gene cluster between the trpCD and trpE genes. Mol Gen Genet. 1983;189(3):375–381. doi: 10.1007/BF00325898. [DOI] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S., Kikuchi A., Kageyama M. Genetic determinant of pyocin R2 in Pseudomonas aeruginosa PAO. II. Physical characterization of pyocin R2 genes using R-prime plasmids constructed from R68.45. Mol Gen Genet. 1983;189(3):382–389. doi: 10.1007/BF00325899. [DOI] [PubMed] [Google Scholar]
- Slilaty S. N., Little J. W. Lysine-156 and serine-119 are required for LexA repressor cleavage: a possible mechanism. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3987–3991. doi: 10.1073/pnas.84.12.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]