Abstract
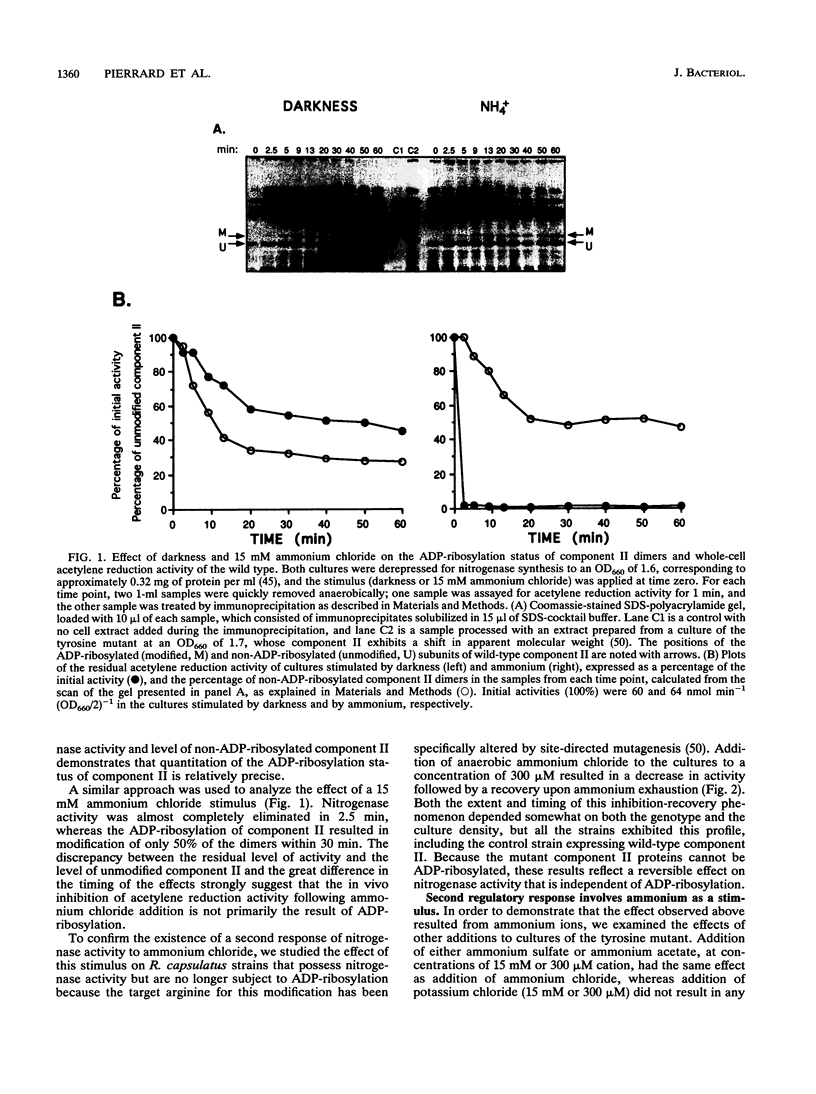
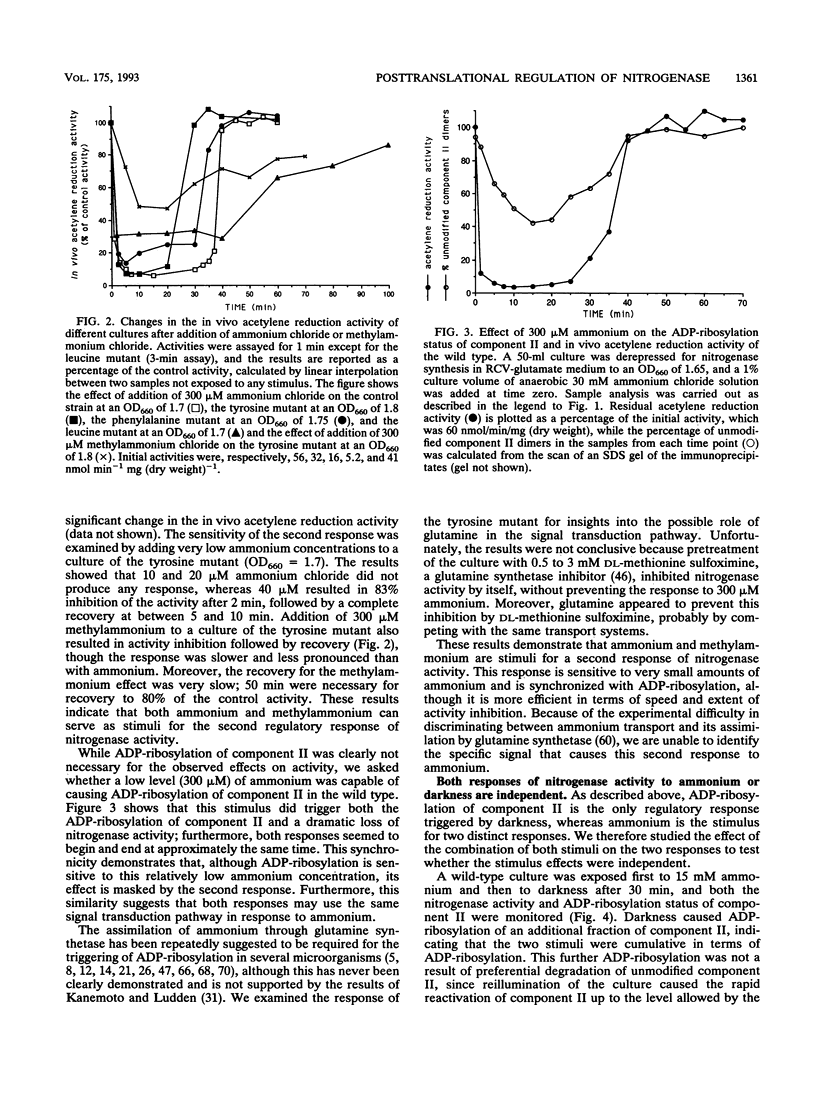
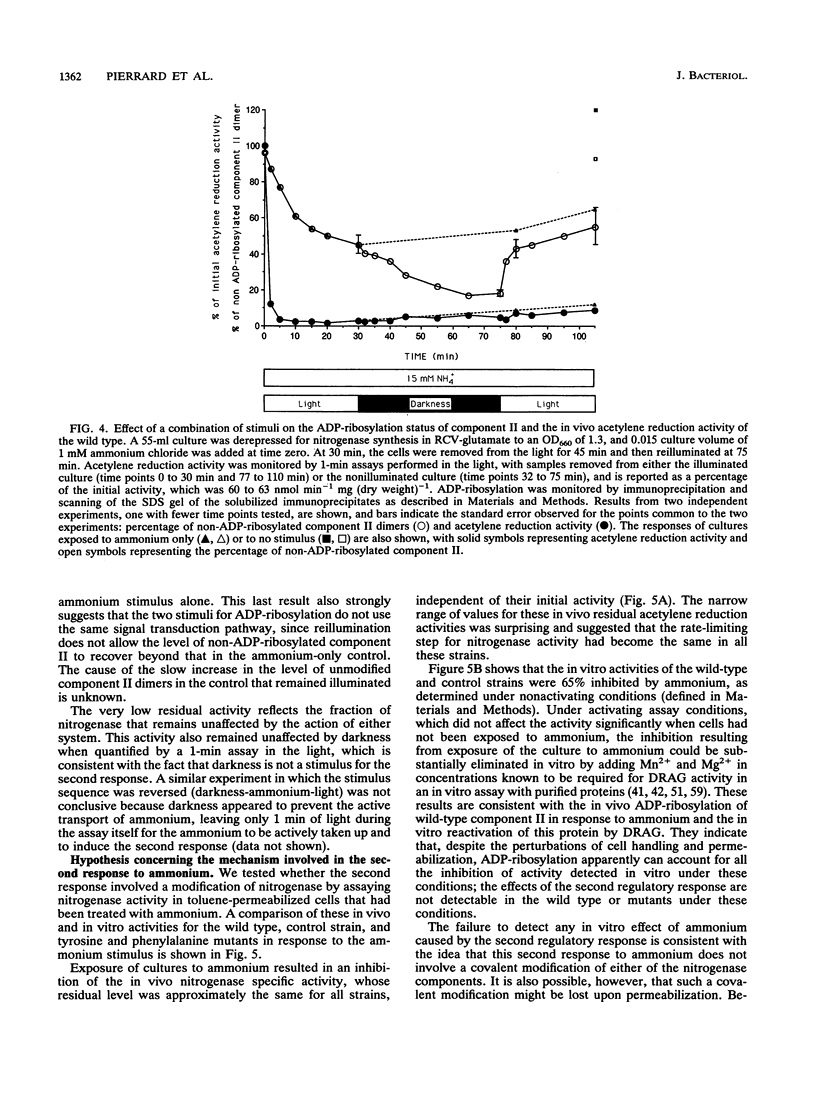
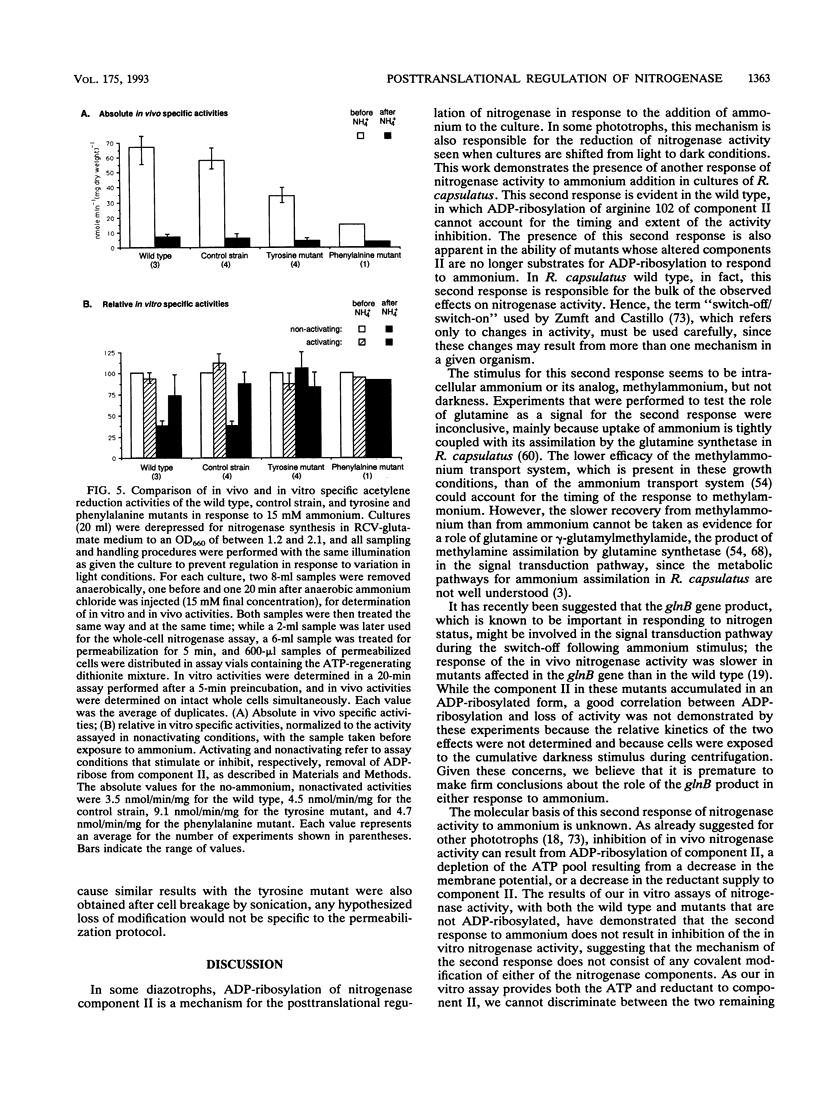
In the photosynthetic bacterium Rhodobacter capsulatus, nitrogenase activity is regulated by ADP-ribosylation of component II in response to the addition of ammonium to cultures or to the removal of light. The ammonium stimulus results in a fast and almost complete inhibition of the in vivo acetylene reduction activity, termed switch-off, which is reversed after the ammonium is exhausted. In the present study of the response of cells to ammonium, ADP-ribosylation of component II occurred but could not account for the extent and timing of the inhibition of activity. The presence of an additional response was confirmed with strains expressing mutant component II proteins; although these proteins are not a substrate for ADP-ribosylation, the strains continued to exhibit a switch-off response to ammonium. This second regulatory response of nitrogenase to ammonium was found to be synchronous with ADP-ribosylation and was responsible for the bulk of the observed effects on nitrogenase activity. In comparison, ADP-ribosylation in R. capsulatus was found to be relatively slow and incomplete but responded independently to both known stimuli, darkness and ammonium. Based on the in vitro nitrogenase activity of both the wild type and strains whose component II proteins cannot be ADP-ribosylated, it seems likely that the second response blocks either the ATP or the electron supply to nitrogenase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bognar A., Desrosiers L., Libman M., Newman E. B. Control of nitrogenase in a photosynthetic autotrophic bacterium, Ectothiorhodospira sp. J Bacteriol. 1982 Nov;152(2):706–713. doi: 10.1128/jb.152.2.706-713.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris R. H. Nitrogenases. J Biol Chem. 1991 May 25;266(15):9339–9342. [PubMed] [Google Scholar]
- Cejudo F. J., de la Torre A., Paneque A. Short-term ammonium inhibition of nitrogen fixation in Azotobacter. Biochem Biophys Res Commun. 1984 Sep 17;123(2):431–437. doi: 10.1016/0006-291x(84)90248-1. [DOI] [PubMed] [Google Scholar]
- Ernst A., Reich S., Böger P. Modification of dinitrogenase reductase in the cyanobacterium Anabaena variabilis due to C starvation and ammonia. J Bacteriol. 1990 Feb;172(2):748–755. doi: 10.1128/jb.172.2.748-755.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992 Jun;56(2):340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice W. P., Saari L. L., Lowery R. G., Ludden P. W., Roberts G. P. Genes coding for the reversible ADP-ribosylation system of dinitrogenase reductase from Rhodospirillum rubrum. Mol Gen Genet. 1989 Aug;218(2):340–347. doi: 10.1007/BF00331287. [DOI] [PubMed] [Google Scholar]
- Foster-Hartnett D., Kranz R. G. Analysis of the promoters and upstream sequences of nifA1 and nifA2 in Rhodobacter capsulatus; activation requires ntrC but not rpoN. Mol Microbiol. 1992 Apr;6(8):1049–1060. doi: 10.1111/j.1365-2958.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- Fu H. A., Fitzmaurice W. P., Roberts G. P., Burris R. H. Cloning and expression of draTG genes from Azospirillum lipoferum. Gene. 1990 Jan 31;86(1):95–98. doi: 10.1016/0378-1119(90)90118-b. [DOI] [PubMed] [Google Scholar]
- Fu H. A., Hartmann A., Lowery R. G., Fitzmaurice W. P., Roberts G. P., Burris R. H. Posttranslational regulatory system for nitrogenase activity in Azospirillum spp. J Bacteriol. 1989 Sep;171(9):4679–4685. doi: 10.1128/jb.171.9.4679-4685.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Burris R. H., Roberts G. P. Reversible ADP-ribosylation is demonstrated to be a regulatory mechanism in prokaryotes by heterologous expression. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1720–1724. doi: 10.1073/pnas.87.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. K., Shah V. K., Brill W. J. Feedback inhibition of nitrogenase. J Bacteriol. 1981 Dec;148(3):884–888. doi: 10.1128/jb.148.3.884-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of nitrogenase activity by covalent modification in Chromatium vinosum. Arch Microbiol. 1985 Feb;141(1):40–43. doi: 10.1007/BF00446737. [DOI] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- Haaker H., Laane C., Hellingwerf K., Houwer B., Konings W. N., Veeger C. Short-term regulation of the nitrogenase activity in Rhodopseudomonas sphaeroides. Eur J Biochem. 1982 Oct;127(3):639–645. doi: 10.1111/j.1432-1033.1982.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Meyer C. M., Vignais P. M. Nitrogenase from the photosynthetic bacterium Rhodopseudomonas capsulata: purification and molecular properties. J Bacteriol. 1982 Feb;149(2):708–717. doi: 10.1128/jb.149.2.708-717.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. C. Mutations affecting nitrogenase switch-off in Rhodobacter capsulatus. Biochim Biophys Acta. 1992 Jan 9;1118(2):161–168. doi: 10.1016/0167-4838(92)90145-4. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Fu H., Burris R. H. Regulation of nitrogenase activity by ammonium chloride in Azospirillum spp. J Bacteriol. 1986 Mar;165(3):864–870. doi: 10.1128/jb.165.3.864-870.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer P., Gest H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: production and utilization of H2 by resting cells. J Bacteriol. 1977 Feb;129(2):732–739. doi: 10.1128/jb.129.2.732-739.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard K. S., Hales B. J., Socolofsky M. D. Nitrogen fixation and ammonia switch-off in the photosynthetic bacterium Rhodopseudomonas viridis. J Bacteriol. 1983 Jul;155(1):107–112. doi: 10.1128/jb.155.1.107-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner P., Willison J. C., Vignais P. M., Bickle T. A. Expression of regulatory nif genes in Rhodobacter capsulatus. J Bacteriol. 1991 May;173(9):2993–2999. doi: 10.1128/jb.173.9.2993-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. L., Monty K. J. Glutamine as a feedback inhibitor of the Rhodopseudomonas sphaeroides nitrogenase system. J Bacteriol. 1979 Sep;139(3):1007–1013. doi: 10.1128/jb.139.3.1007-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Haselkorn R. The DNA sequence of the Rhodobacter capsulatus ntrA, ntrB and ntrC gene analogues required for nitrogen fixation. Mol Gen Genet. 1989 Feb;215(3):507–516. doi: 10.1007/BF00427050. [DOI] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Amino acid concentrations in Rhodospirillum rubrum during expression and switch-off of nitrogenase activity. J Bacteriol. 1987 Jul;169(7):3035–3043. doi: 10.1128/jb.169.7.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugkist J., Haaker H. Inhibition of nitrogenase activity by ammonium chloride in Azotobacter vinelandii. J Bacteriol. 1984 Jan;157(1):148–151. doi: 10.1128/jb.157.1.148-151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz W. G., LaRue T. A., Chatson K. B. Nitrogenase in synchronized Azotobacter vinelandii OP. Can J Microbiol. 1975 Jul;21(7):984–988. doi: 10.1139/m75-145. [DOI] [PubMed] [Google Scholar]
- Laane C., Krone W., Konings W., Haaker H., Veeger C. Short-term effect of ammonium chloride on nitrogen fixation by Azotobacter vinelandii and by bacteroids of Rhizobium leguminosarum. Eur J Biochem. 1980 Jan;103(1):39–46. doi: 10.1111/j.1432-1033.1980.tb04286.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang J. H., Nielsen G. M., Lies D. P., Burris R. H., Roberts G. P., Ludden P. W. Mutations in the draT and draG genes of Rhodospirillum rubrum result in loss of regulation of nitrogenase by reversible ADP-ribosylation. J Bacteriol. 1991 Nov;173(21):6903–6909. doi: 10.1128/jb.173.21.6903-6909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo A. L., Zinder S. H. Nitrogenase in the archaebacterium Methanosarcina barkeri 227. J Bacteriol. 1990 Dec;172(12):6789–6796. doi: 10.1128/jb.172.12.6789-6796.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery R. G., Ludden P. W. Purification and properties of dinitrogenase reductase ADP-ribosyltransferase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1988 Nov 15;263(32):16714–16719. [PubMed] [Google Scholar]
- Lowery R. G., Saari L. L., Ludden P. W. Reversible regulation of the nitrogenase iron protein from Rhodospirillum rubrum by ADP-ribosylation in vitro. J Bacteriol. 1986 May;166(2):513–518. doi: 10.1128/jb.166.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W. Borate inhibits activation of inactive dinitrogenase reductase from Rhodospirillum rubrum. Biochem J. 1981 Aug 1;197(2):503–505. doi: 10.1042/bj1970503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Removal of an adenine-like molecule during activation of dinitrogenase reductase from Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6201–6205. doi: 10.1073/pnas.76.12.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Roberts G. P. Regulation of nitrogenase activity by reversible ADP ribosylation. Curr Top Cell Regul. 1989;30:23–56. doi: 10.1016/b978-0-12-152830-0.50004-9. [DOI] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Kelley B. C., Vignais P. M. Effect of light nitrogenase function and synthesis in Rhodopseudomonas capsulata. J Bacteriol. 1978 Oct;136(1):201–208. doi: 10.1128/jb.136.1.201-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Vignais P. M. Effects of L-methionine-DL-sulfoximine and beta-N-oxalyl-L-alpha, beta-diaminopropionic acid on nitrogenase biosynthesis and activity in Rhodopseudomonas capsulata. Biochem Biophys Res Commun. 1979 Jul 27;89(2):353–359. doi: 10.1016/0006-291x(79)90637-5. [DOI] [PubMed] [Google Scholar]
- Pope M. R., Saari L. L., Ludden P. W. N-glycohydrolysis of adenosine diphosphoribosyl arginine linkages by dinitrogenase reductase activating glycohydrolase (activating enzyme) from Rhodospirillum rubrum. J Biol Chem. 1986 Aug 5;261(22):10104–10111. [PubMed] [Google Scholar]
- Preker P., Hübner P., Schmehl M., Klipp W., Bickle T. A. Mapping and characterization of the promoter elements of the regulatory nif genes rpoN, nifA1 and nifA2 in Rhodobacter capsulatus. Mol Microbiol. 1992 Apr;6(8):1035–1047. doi: 10.1111/j.1365-2958.1992.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Saari L. L., Triplett E. W., Ludden P. W. Purification and properties of the activating enzyme for iron protein of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1984 Dec 25;259(24):15502–15508. [PubMed] [Google Scholar]
- Smith R. L., Van Baalen C., Tabita F. R. Control of nitrogenase recovery from oxygen inactivation by ammonia in the cyanobacterium Anabaena sp. strain CA (ATCC 33047). J Bacteriol. 1990 May;172(5):2788–2790. doi: 10.1128/jb.172.5.2788-2790.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. D., Hartmann A., Burris R. H. Purification and properties of the nitrogenase of Azospirillum amazonense. J Bacteriol. 1985 Dec;164(3):1271–1277. doi: 10.1128/jb.164.3.1271-1277.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Gest H. Derepression of nitrogenase activity in glutamine auxotrophs of Rhodopseudomonas capsulata. J Bacteriol. 1979 Mar;137(3):1459–1463. doi: 10.1128/jb.137.3.1459-1463.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Li J. D., Hu C. Z., Scholin C. Ammonia switch-off of nitrogenase from Rhodobacter sphaeroides and Methylosinus trichosporium: no evidence for Fe protein modification. Arch Microbiol. 1988 May;150(1):1–5. doi: 10.1007/BF00409708. [DOI] [PubMed] [Google Scholar]
- Yoch D. C. Regulation of nitrogenase A and R concentrations in Rhodopseudomonas capsulata by glutamine synthetase. Biochem J. 1980 Apr 1;187(1):273–276. doi: 10.1042/bj1870273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Zhang Z. M., Claybrook D. L. Methylamine metabolism and its role in nitrogenase "switch off" in Rhodopseudomonas capsulata. Arch Microbiol. 1983 Jan;134(1):45–48. doi: 10.1007/BF00429405. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Burris R. H., Roberts G. P. Cloning, sequencing, mutagenesis, and functional characterization of draT and draG genes from Azospirillum brasilense. J Bacteriol. 1992 May;174(10):3364–3369. doi: 10.1128/jb.174.10.3364-3369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978 Apr 27;117(1):53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]



