Abstract
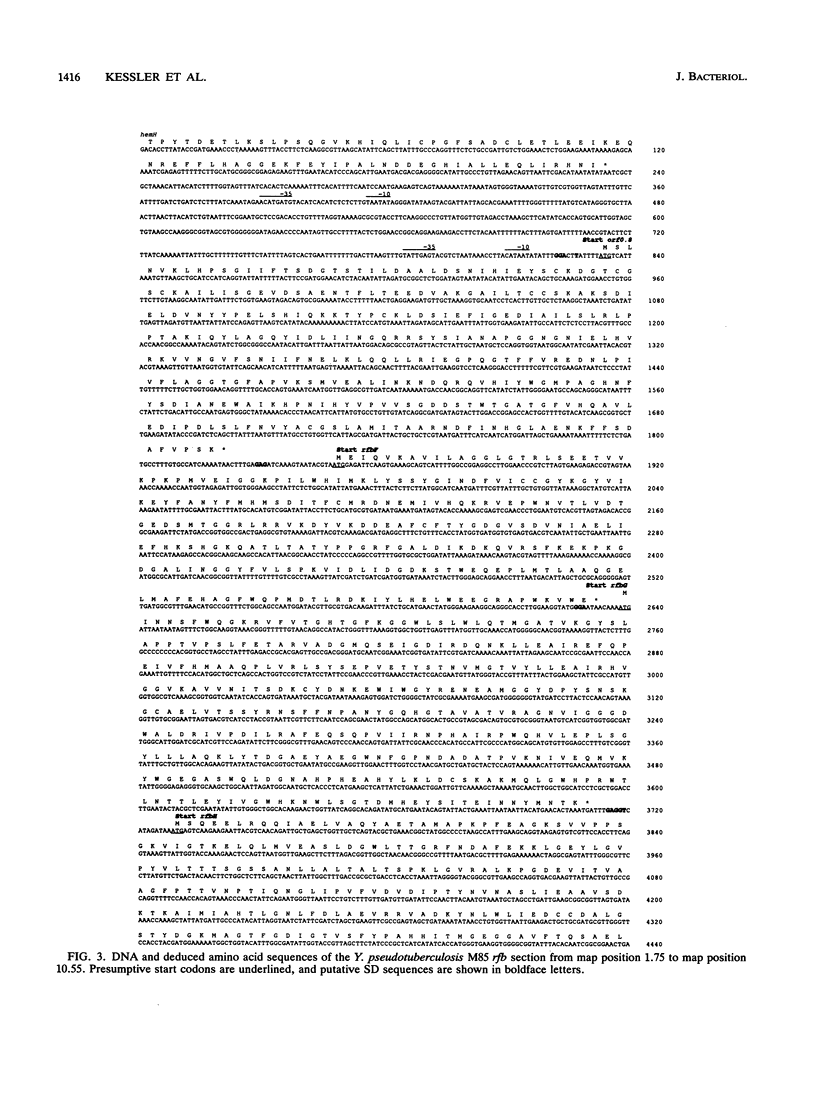
Salmonella enterica and Yersinia pseudotuberculosis are the only examples in nature known to use a variety of 3,6-dideoxyhexose derivatives as O antigen constituents. To allow a comparison of the responsible biosynthetic genes of the two organisms, we have sequenced a section of the Y. pseudotuberculosis serogroup IIA rfb region that contained the genes for the abequose biosynthetic pathway. Comparison of the identified genes with the rfb region of S. enterica LT2 showed that the two dideoxyhexose pathway gene clusters are related. The arrangement of the genes was largely conserved, and the G + C compositions of the two DNA regions were strikingly similar; however, the degree of conservation of nucleotide and protein sequences suggested that the two gene clusters have been evolving independently for considerable time. Hybridization experiments showed that the dideoxyhexose pathway genes are widespread throughout the various serogroups of Y. pseudotuberculosis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksić S., Suchan G., Bockemühl J., Aleksić V. An extended antigenic scheme for Yersinia pseudotuberculosis. Contrib Microbiol Immunol. 1991;12:235–238. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Wolf R. E., Jr Growth-rate-dependent expression and cloning of gnd alleles from natural isolates of Escherichia coli. J Bacteriol. 1988 Jan;170(1):365–371. doi: 10.1128/jb.170.1.365-371.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin D. A., Romana L. K., Reeves P. R. Molecular cloning and expression in Escherichia coli K-12 of the rfb gene cluster determining the O antigen of an E. coli O111 strain. Mol Microbiol. 1991 Sep;5(9):2223–2231. doi: 10.1111/j.1365-2958.1991.tb02152.x. [DOI] [PubMed] [Google Scholar]
- Blondel A., Thillet J. A fast and convenient way to produce single stranded DNA from a phagemid. Nucleic Acids Res. 1991 Jan 11;19(1):181–181. doi: 10.1093/nar/19.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. K., Romana L. K., Reeves P. R. Cloning of the rfb gene cluster of a group C2 Salmonella strain: comparison with the rfb regions of groups B and D. Mol Microbiol. 1991 Aug;5(8):1873–1881. doi: 10.1111/j.1365-2958.1991.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Brown P. K., Romana L. K., Reeves P. R. Molecular analysis of the rfb gene cluster of Salmonella serovar muenchen (strain M67): the genetic basis of the polymorphism between groups C2 and B. Mol Microbiol. 1992 May;6(10):1385–1394. doi: 10.1111/j.1365-2958.1992.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Ryu J. I. Salmonella typhimurium LT2 strains which are r- m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983 Oct;156(1):471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. II. GUANOSINE DIPHOSPHATE 4-KETO-6-DEOXY-D-MANNOSE, AN INTERMEDIATE IN THE BIOSYNTHESIS OF GUANOSINE DIPHOSPHATE COLITOSE. J Biol Chem. 1965 May;240:1926–1931. [PubMed] [Google Scholar]
- Gorshkova R. P., Zubkov V. A., Isakov V. V., Ovodov Iu S. Struktura O-spetsificheskogo polisakharida iz lipopolisakharida Yersinia pseudotuberculosis VI serovara. Bioorg Khim. 1983 Aug;9(8):1068–1073. [PubMed] [Google Scholar]
- Han O., Miller V. P., Liu H. W. Mechanistic studies of the biosynthesis of 3,6-dideoxyhexoses in Yersinia pseudotuberculosis. Purification and characterization of CDP-6-deoxy-delta 3,4-glucoseen reductase based on its NADH:dichlorophenolindolphenol oxidoreductase activity. J Biol Chem. 1990 May 15;265(14):8033–8041. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Jiang X. M., Neal B., Santiago F., Lee S. J., Romana L. K., Reeves P. R. Structure and sequence of the rfb (O antigen) gene cluster of Salmonella serovar typhimurium (strain LT2). Mol Microbiol. 1991 Mar;5(3):695–713. doi: 10.1111/j.1365-2958.1991.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. I. Los Alamos sequence analysis package for nucleic acids and proteins. Nucleic Acids Res. 1982 Jan 11;10(1):183–196. doi: 10.1093/nar/10.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A. C., Brown P. K., Romana L. K., Reeves P. R. Molecular cloning and genetic characterization of the rfb region from Yersinia pseudotuberculosis serogroup IIA, which determines the formation of the 3,6-dideoxyhexose abequose. J Gen Microbiol. 1991 Dec;137(12):2689–2695. doi: 10.1099/00221287-137-12-2689. [DOI] [PubMed] [Google Scholar]
- Kotandrova N. A., Gorshkova R. P., Zubkov V. A., Ovodov Iu S. Stroenie O-spetsificheskoi polisakharidnoi tsepi lipopolisakharida Yersinia pseudotuberculosis serovara VII. Bioorg Khim. 1989 Jan;15(1):104–110. [PubMed] [Google Scholar]
- Lee S. J., Romana L. K., Reeves P. R. Cloning and structure of group C1 O antigen (rfb gene cluster) from Salmonella enterica serovar montevideo. J Gen Microbiol. 1992 Feb;138(2):305–312. doi: 10.1099/00221287-138-2-305. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Romana L. K., Reeves P. R. Sequence and structural analysis of the rfb (O antigen) gene cluster from a group C1 Salmonella enterica strain. J Gen Microbiol. 1992 Sep;138(9):1843–1855. doi: 10.1099/00221287-138-9-1843. [DOI] [PubMed] [Google Scholar]
- Liu D., Verma N. K., Romana L. K., Reeves P. R. Relationships among the rfb regions of Salmonella serovars A, B, and D. J Bacteriol. 1991 Aug;173(15):4814–4819. doi: 10.1128/jb.173.15.4814-4819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi S., Matsuhashi M., Strominger J. L. Enzymatic synthesis of cytidine diphosphate 3,6-dideoxyhexoses. I. Over-all reactions. J Biol Chem. 1966 Sep 25;241(18):4267–4274. [PubMed] [Google Scholar]
- Miyamoto K., Nakahigashi K., Nishimura K., Inokuchi H. Isolation and characterization of visible light-sensitive mutants of Escherichia coli K12. J Mol Biol. 1991 Jun 5;219(3):393–398. doi: 10.1016/0022-2836(91)90180-e. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- O'Neill M. C., Chiafari F. Escherichia coli promoters. II. A spacing class-dependent promoter search protocol. J Biol Chem. 1989 Apr 5;264(10):5531–5534. [PubMed] [Google Scholar]
- O'Neill M. C. Consensus methods for finding and ranking DNA binding sites. Application to Escherichia coli promoters. J Mol Biol. 1989 May 20;207(2):301–310. doi: 10.1016/0022-2836(89)90256-8. [DOI] [PubMed] [Google Scholar]
- Samuelsson K., Lindberg B., Brubaker R. R. Structure of O-specific side chains of lipopolysaccharides from Yersinia pseudotuberculosis. J Bacteriol. 1974 Mar;117(3):1010–1016. doi: 10.1128/jb.117.3.1010-1016.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. C. Method to determine the reading frame of a protein from the purine/pyrimidine genome sequence and its possible evolutionary justification. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1596–1600. doi: 10.1073/pnas.78.3.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. Directional mutation pressure and neutral molecular evolution. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2653–2657. doi: 10.1073/pnas.85.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N. K., Quigley N. B., Reeves P. R. O-antigen variation in Salmonella spp.: rfb gene clusters of three strains. J Bacteriol. 1988 Jan;170(1):103–107. doi: 10.1128/jb.170.1.103-107.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N., Reeves P. Identification and sequence of rfbS and rfbE, which determine antigenic specificity of group A and group D salmonellae. J Bacteriol. 1989 Oct;171(10):5694–5701. doi: 10.1128/jb.171.10.5694-5701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wang L., Romana L. K., Reeves P. R. Molecular analysis of a Salmonella enterica group E1 rfb gene cluster: O antigen and the genetic basis of the major polymorphism. Genetics. 1992 Mar;130(3):429–443. doi: 10.1093/genetics/130.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel T. M., Liu L. D., Liu H. W. Mechanistic studies of the biosynthesis of 3,6-dideoxyhexoses in Yersinia pseudotuberculosis: purification and characterization of CDP-4-keto-6-deoxy-D-glucose-3-dehydrase. Biochemistry. 1992 Feb 25;31(7):2129–2139. doi: 10.1021/bi00122a034. [DOI] [PubMed] [Google Scholar]
- Wyk P., Reeves P. Identification and sequence of the gene for abequose synthase, which confers antigenic specificity on group B salmonellae: homology with galactose epimerase. J Bacteriol. 1989 Oct;171(10):5687–5693. doi: 10.1128/jb.171.10.5687-5693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


