Abstract
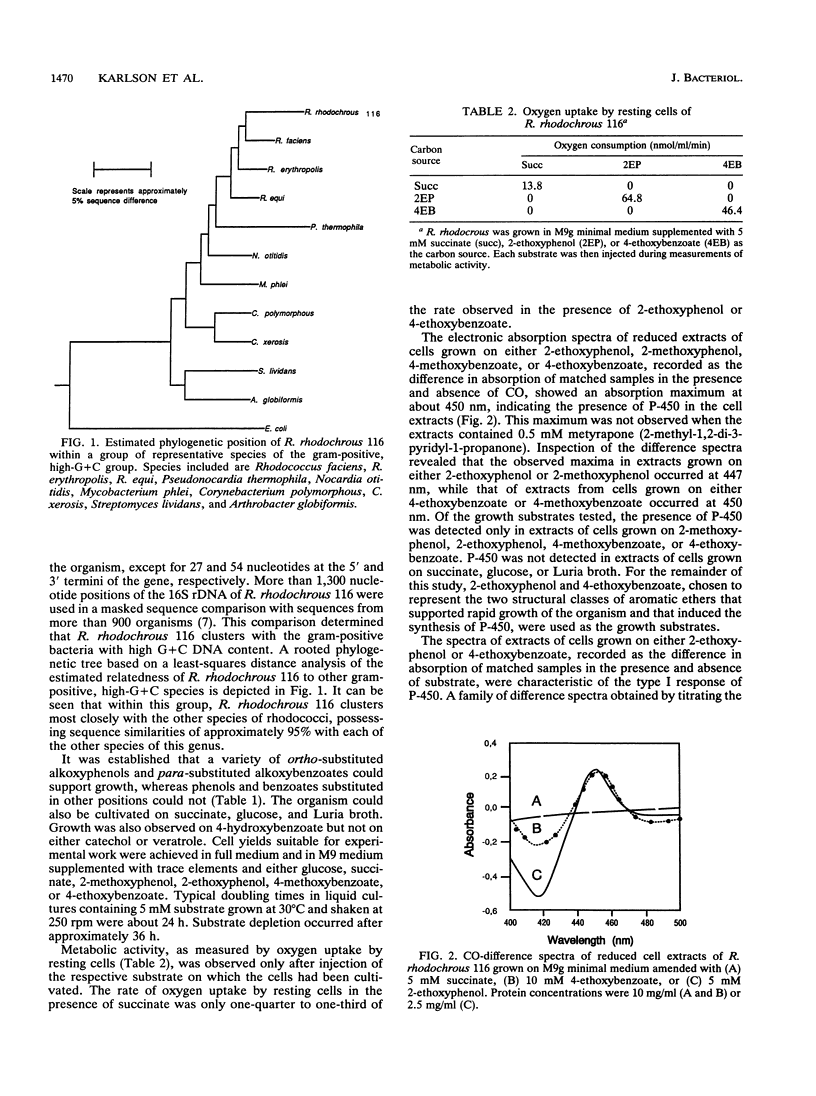
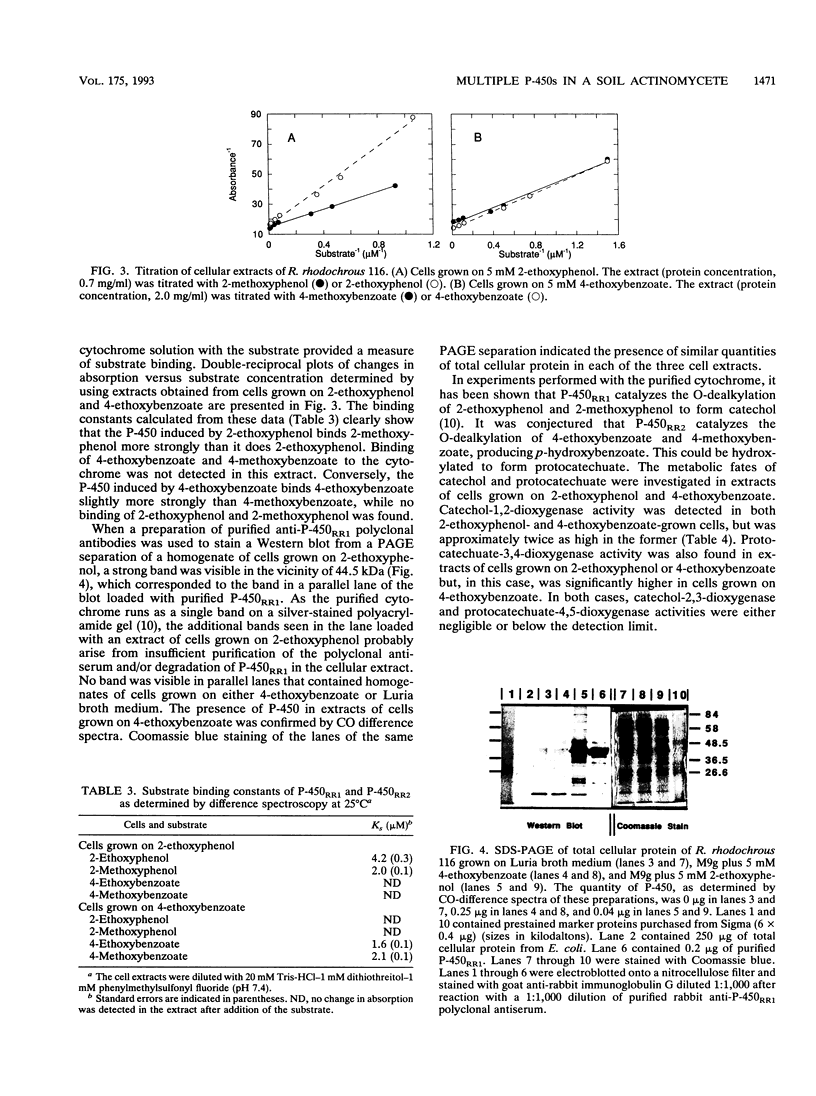
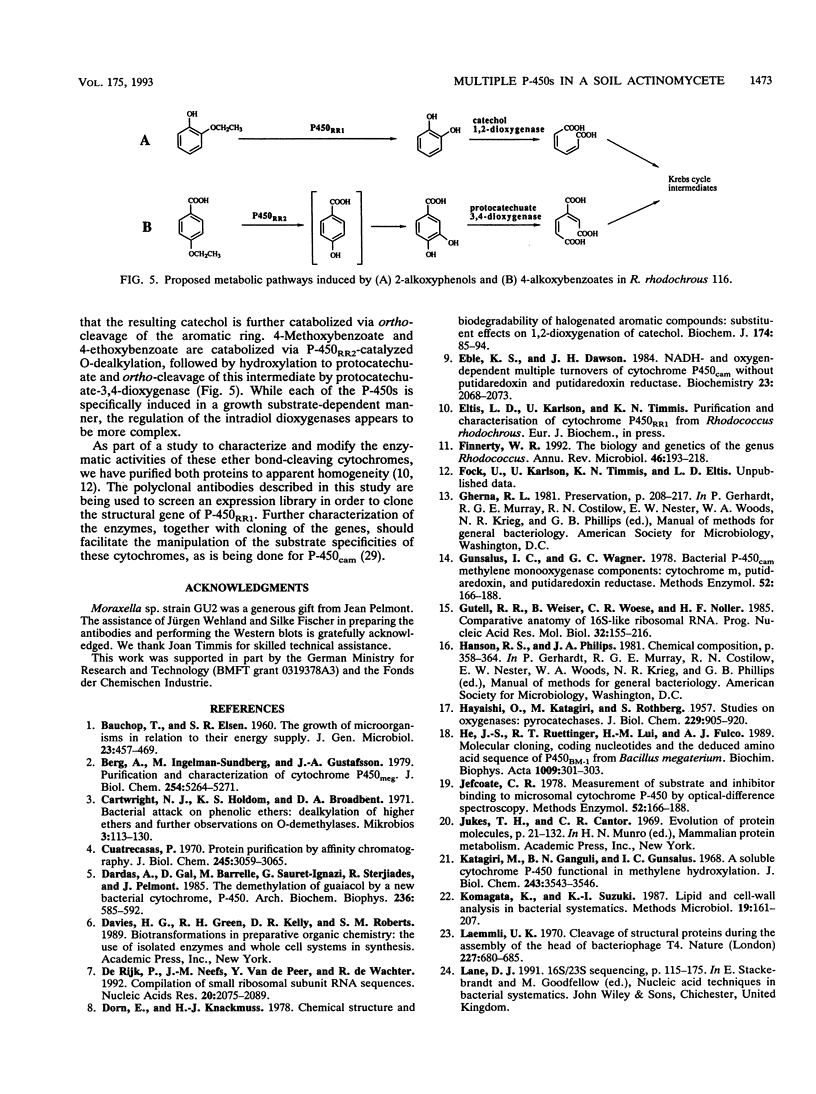
A red-pigmented coryneform bacterium, identified as Rhodococcus rhodochrous strain 116, that grew on 2-ethoxyphenol and 4-methoxybenzoate as sole carbon and energy sources was isolated. Phylogenetic analysis based on the 16S rDNA sequences indicates that the strain clusters more closely to other rhodococci than to other gram-positive organisms with a high G + C content. Each of the abovementioned growth substrates was shown to induce a distinct cytochrome P-450: cytochrome P-450RR1 was induced by 2-ethoxyphenol, and cytochrome P-450RR2 was induced by 4-methoxybenzoate. A type I difference spectrum typical of substrate binding was induced in cytochrome P-450RR1 by both 2-ethoxyphenol (KS = 4.2 +/- 0.3 microM) and 2-methoxyphenol (KS = 2.0 +/- 0.1 microM), but not 4-methoxybenzoate or 4-ethoxybenzoate. Similarly, a type I difference spectrum was induced in cytochrome P-450RR2 by both 4-methoxybenzoate (KS = 2.1 +/- 0.1 microM) and 4-ethoxybenzoate (KS = 1.6 +/- 0.1 microM), but not 2-methoxyphenol or 2-ethoxyphenol. A purified polyclonal antiserum prepared against cytochrome P-450RR1 did not cross-react with cytochrome P-450RR2, indicating that the proteins are immunologically distinct. The cytochromes appear to catalyze the O-dealkylation of their respective substrates. The respective products of the O-dealkylation are further metabolized via ortho cleavage enzymes, whose expression is also regulated by the respective aromatic ethers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Berg A., Ingelman-Sundberg M., Gustafsson J. A. Purification and characterization of cytochrome P-450meg. J Biol Chem. 1979 Jun 25;254(12):5264–5271. [PubMed] [Google Scholar]
- Cartwright N. J., Holdom K. S., Broadbent D. A. Bacterial attack on phenolic ethers. Dealkylation of higher ethers and further observations on O-demethylases. Microbios. 1971 Mar;3(10):113–130. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dardas A., Gal D., Barrelle M., Sauret-Ignazi G., Sterjiades R., Pelmont J. The demethylation of guaiacol by a new bacterial cytochrome P-450. Arch Biochem Biophys. 1985 Feb 1;236(2):585–592. doi: 10.1016/0003-9861(85)90662-9. [DOI] [PubMed] [Google Scholar]
- De Rijk P., Neefs J. M., Van de Peer Y., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1992 May 11;20 (Suppl):2075–2089. doi: 10.1093/nar/20.suppl.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble K. S., Dawson J. H. NADH- and oxygen-dependent multiple turnovers of cytochrome P-450-CAM without putidaredoxin and putidaredoxin reductase. Biochemistry. 1984 Apr 24;23(9):2068–2073. doi: 10.1021/bi00304a029. [DOI] [PubMed] [Google Scholar]
- Finnerty W. R. The biology and genetics of the genus Rhodococcus. Annu Rev Microbiol. 1992;46:193–218. doi: 10.1146/annurev.mi.46.100192.001205. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Wagner G. C. Bacterial P-450cam methylene monooxygenase components: cytochrome m, putidaredoxin, and putidaredoxin reductase. Methods Enzymol. 1978;52:166–188. doi: 10.1016/s0076-6879(78)52019-3. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Wagner G. C. Bacterial P-450cam methylene monooxygenase components: cytochrome m, putidaredoxin, and putidaredoxin reductase. Methods Enzymol. 1978;52:166–188. doi: 10.1016/s0076-6879(78)52019-3. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- HAYAISHI O., KATAGIRI M., ROTHBERG S. Studies on oxygenases; pyrocatechase. J Biol Chem. 1957 Dec;229(2):905–920. [PubMed] [Google Scholar]
- He J. S., Ruettinger R. T., Liu H. M., Fulco A. J. Molecular cloning, coding nucleotides and the deduced amino acid sequence of P-450BM-1 from Bacillus megaterium. Biochim Biophys Acta. 1989 Dec 22;1009(3):301–303. doi: 10.1016/0167-4781(89)90120-6. [DOI] [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakai C., Nakazawa T., Nozaki M. Purification and properties of catechol 1,2-dioxygenase (pyrocatechase) from Pseudomonas putida mt-2 in comparison with that from Pseudomonas arvilla C-1. Arch Biochem Biophys. 1988 Dec;267(2):701–713. doi: 10.1016/0003-9861(88)90079-3. [DOI] [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986 Jun 5;261(16):7160–7169. [PubMed] [Google Scholar]
- Olsen G. J. Earliest phylogenetic branchings: comparing rRNA-based evolutionary trees inferred with various techniques. Cold Spring Harb Symp Quant Biol. 1987;52:825–837. doi: 10.1101/sqb.1987.052.01.090. [DOI] [PubMed] [Google Scholar]
- Peterson J. A., Lu J. Y. Bacterial cytochromes P450: isolation and identification. Methods Enzymol. 1991;206:612–620. doi: 10.1016/0076-6879(91)06131-l. [DOI] [PubMed] [Google Scholar]
- Petzold D. R., Rein H., Schwarz D., Sommer M., Ruckpaul K. Relation between the structure of benzphetamine analogues and their binding properties to cytochrome P-450 LM2. Biochim Biophys Acta. 1985 Jun 10;829(2):253–261. doi: 10.1016/0167-4838(85)90195-5. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., O'Keefe D. P. Induction of cytochrome P-450-dependent sulfonylurea metabolism in Streptomyces griseolus. Biochem Biophys Res Commun. 1986 Oct 30;140(2):650–659. doi: 10.1016/0006-291x(86)90781-3. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., INGRAHAM J. L. Protocatechuic acid oxidase. J Biol Chem. 1954 Oct;210(2):799–808. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S. Microbial cytochromes P-450 and xenobiotic metabolism. Adv Appl Microbiol. 1991;36:133–178. doi: 10.1016/s0065-2164(08)70453-2. [DOI] [PubMed] [Google Scholar]
- Sterjiades R., Pelmont J. Occurrence of two different forms of protocatechuate 3,4-dioxygenase in a Moraxella sp. Appl Environ Microbiol. 1989 Feb;55(2):340–347. doi: 10.1128/aem.55.2.340-347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. B. Demethylation of Veratrole by Cytochrome P-450 in Streptomyces setonii. Appl Environ Microbiol. 1986 Jul;52(1):98–100. doi: 10.1128/aem.52.1.98-100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A. J., Murray R. I., Bhattacharyya P. K., Wagner G. C., Gunsalus I. C. Protein components of a cytochrome P-450 linalool 8-methyl hydroxylase. J Biol Chem. 1990 Jan 25;265(3):1345–1351. [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



