Abstract
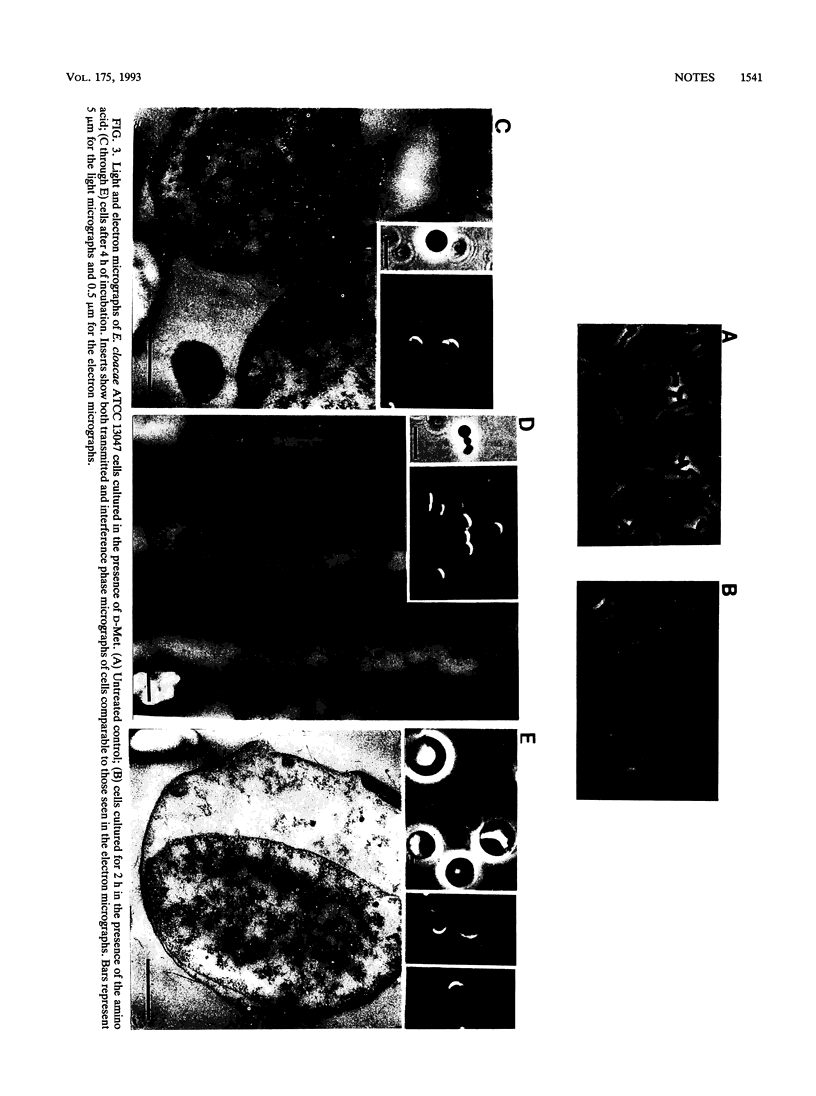
Induction of AmpC beta-lactamase in Enterobacter cloacae ATCC 13047 by D-methionine, glycine, or D-tryptophan was accompanied by alterations in peptidoglycan composition and structure; in the case of D-methionine, it was also accompanied by morphologic changes. A decrease in peptidoglycan tripeptides was seen. With glycine, there was an increase in the proportion of diaminopimelic-diaminopimelic cross-links. The possible implications of these changes for beta-lactamase induction are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg K. J., Takasuga A., Edwards D. H., Dewar S. J., Spratt B. G., Adachi H., Ohta T., Matsuzawa H., Donachie W. D. The balance between different peptidoglycan precursors determines whether Escherichia coli cells will elongate or divide. J Bacteriol. 1990 Dec;172(12):6697–6703. doi: 10.1128/jb.172.12.6697-6703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caparrós M., Pisabarro A. G., de Pedro M. A. Effect of D-amino acids on structure and synthesis of peptidoglycan in Escherichia coli. J Bacteriol. 1992 Sep;174(17):5549–5559. doi: 10.1128/jb.174.17.5549-5559.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W., Dalhoff A., Dick W. Nonspecific induction of beta-lactamase in Enterobacter cloacae. J Gen Microbiol. 1984 Jul;130(7):1781–1786. doi: 10.1099/00221287-130-7-1781. [DOI] [PubMed] [Google Scholar]
- Dalhoff A., Cullmann W. Specificity of beta-lactamase induction in Pseudomonas aeruginosa. J Antimicrob Chemother. 1984 Oct;14(4):349–357. doi: 10.1093/jac/14.4.349. [DOI] [PubMed] [Google Scholar]
- Gatus B. J., Bell S. M., Jimenez A. S. Comparison of glycine enhancement with cefoxitin induction of class 1 beta-lactamase production in Enterobacter cloacae ATCC 13047. J Antimicrob Chemother. 1988 Feb;21(2):163–170. doi: 10.1093/jac/21.2.163. [DOI] [PubMed] [Google Scholar]
- Gatus B. J., Bell S. M., Jimenez A. S. Enhancement of beta-lactamase production by glycine in Enterobacter cloacae ATCC.13047. Pathology. 1986 Jan;18(1):145–147. doi: 10.3109/00313028609090843. [DOI] [PubMed] [Google Scholar]
- Glauner B., Höltje J. V. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18988–18996. [PubMed] [Google Scholar]
- Hammes W., Schleifer K. H., Kandler O. Mode of action of glycine on the biosynthesis of peptidoglycan. J Bacteriol. 1973 Nov;116(2):1029–1053. doi: 10.1128/jb.116.2.1029-1053.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK C., LARK K. G. The effects of D-amino acids on Alcaligenes fecalis. Can J Microbiol. 1959 Aug;5:369–379. doi: 10.1139/m59-046. [DOI] [PubMed] [Google Scholar]
- Letendre E. D., Turgeon P. L. Production and induction of beta-lactamase during growth of Pseudomonas aeruginosa in biological fluids. Antimicrob Agents Chemother. 1989 May;33(5):776–777. doi: 10.1128/aac.33.5.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi A. C., Ayala J. A. Induction of a class I beta-lactamase from Citrobacter freundii in Escherichia coli requires active ftsZ but not ftsA or ftsQ products. Antimicrob Agents Chemother. 1991 Nov;35(11):2359–2365. doi: 10.1128/aac.35.11.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisabarro A. G., de Pedro M. A., Vázquez D. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol. 1985 Jan;161(1):238–242. doi: 10.1128/jb.161.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Tsuruoka T., Tamura A., Miyata A., Takei T., Iwamatsu K., Inouye S., Matsuhashi M. Penicillin-insensitive incorporation of D-amino acids into cell wall peptidoglycan influences the amount of bound lipoprotein in Escherichia coli. J Bacteriol. 1984 Dec;160(3):889–894. doi: 10.1128/jb.160.3.889-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]