Abstract
The SecB, SecA, and SecY dependency of a small outer membrane lipoprotein in Escherichia coli, the bacteriocin release protein (BRP), was studied. The detrimental effect of BRP expression on the culture turbidity (quasi-lysis) was strongly reduced in the sec mutants. Immunoblotting and radioactive labeling experiments showed that the expression, membrane insertion, and processing of the BRP precursor are dependent on SecB, SecA, and SecY. Labeling experiments with hybrid BRP gene constructs revealed that the mature part of the BRP precursor and not its stable signal sequence is important for its SecB dependency.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage L., Hendrick J. P., Schiebel E., Driessen A. J., Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990 Aug 24;62(4):649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- Cabelli R. J., Chen L., Tai P. C., Oliver D. B. SecA protein is required for secretory protein translocation into E. coli membrane vesicles. Cell. 1988 Nov 18;55(4):683–692. doi: 10.1016/0092-8674(88)90227-9. [DOI] [PubMed] [Google Scholar]
- Cavard D., Baty D., Howard S. P., Verheij H. M., Lazdunski C. Lipoprotein nature of the colicin A lysis protein: effect of amino acid substitutions at the site of modification and processing. J Bacteriol. 1987 May;169(5):2187–2194. doi: 10.1128/jb.169.5.2187-2194.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D. Colicin A and colicin E1 lysis proteins differ in their dependence on secA and secY gene products. FEBS Lett. 1992 Feb 17;298(1):84–88. doi: 10.1016/0014-5793(92)80027-e. [DOI] [PubMed] [Google Scholar]
- Cobet W. W., Mollay C., Müller G., Zimmermann R. Export of honeybee prepromelittin in Escherichia coli depends on the membrane potential but does not depend on proteins secA and secY. J Biol Chem. 1989 Jun 15;264(17):10169–10176. [PubMed] [Google Scholar]
- De Graaf F. K., Oudega B. Production and release of cloacin DF13 and related colicins. Curr Top Microbiol Immunol. 1986;125:183–205. doi: 10.1007/978-3-642-71251-7_11. [DOI] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Degen M., Henning U. A lower size limit exists for export of fragments of an outer membrane protein (OmpA) of Escherichia coli K-12. J Mol Biol. 1989 Feb 20;205(4):771–775. doi: 10.1016/0022-2836(89)90321-5. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Lecker S., Schiebel E., Hendrick J. P., Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990 Oct 19;63(2):269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- Kuhn A. Alterations in the extracellular domain of M13 procoat protein make its membrane insertion dependent on secA and secY. Eur J Biochem. 1988 Nov 1;177(2):267–271. doi: 10.1111/j.1432-1033.1988.tb14372.x. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Kreil G., Wickner W. Recombinant forms of M13 procoat with an OmpA leader sequence or a large carboxy-terminal extension retain their independence of secY function. EMBO J. 1987 Feb;6(2):501–505. doi: 10.1002/j.1460-2075.1987.tb04781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
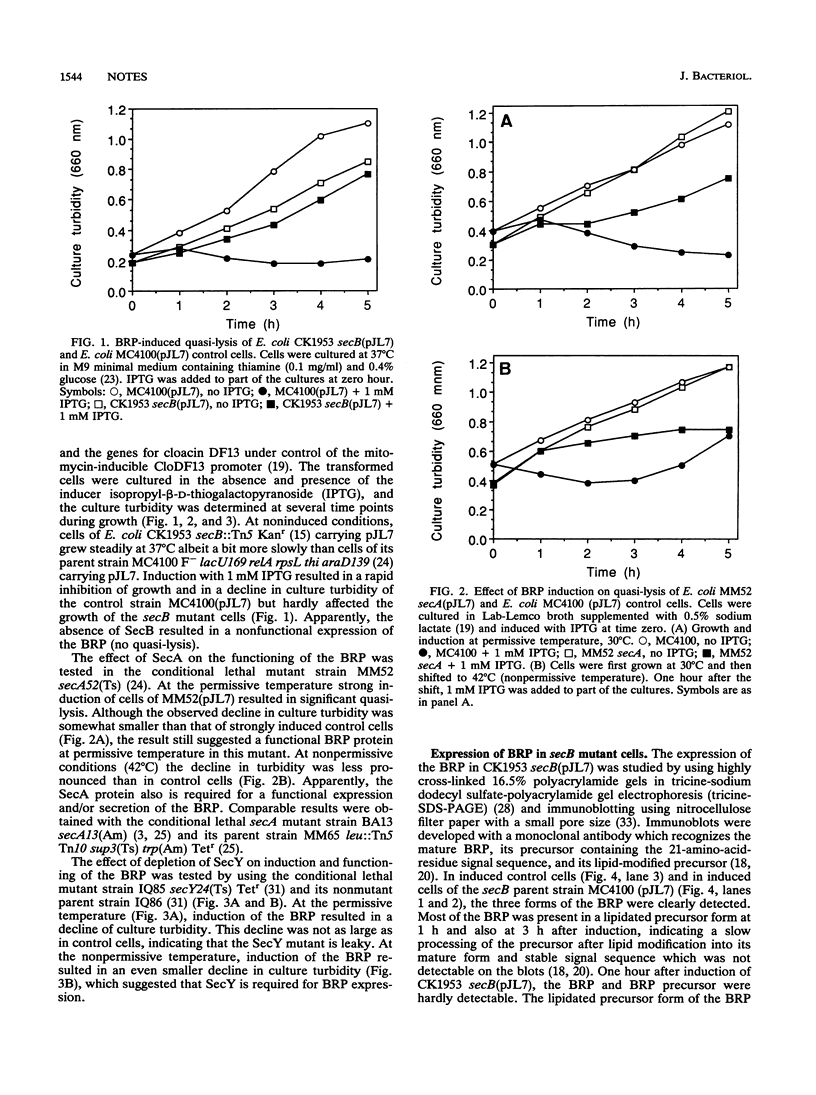
- Kumamoto C. A., Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985 Jul;163(1):267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5320–5324. doi: 10.1073/pnas.86.14.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Gannon P. M. Effects of Escherichia coli secB mutations on pre-maltose binding protein conformation and export kinetics. J Biol Chem. 1988 Aug 15;263(23):11554–11558. [PubMed] [Google Scholar]
- Lill R., Cunningham K., Brundage L. A., Ito K., Oliver D., Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989 Mar;8(3):961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., Clark D. M., Ras J., Verschoor E. J., Stegehuis F., de Graaf F. K., Oudega B. pCloDF13-encoded bacteriocin release proteins with shortened carboxyl-terminal segments are lipid modified and processed and function in release of cloacin DF13 and apparent host cell lysis. J Bacteriol. 1989 May;171(5):2673–2679. doi: 10.1128/jb.171.5.2673-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., Duim B., de Gier J. W., Oudega B. Functioning of the stable signal peptide of the pCloDF13-encoded bacteriocin release protein. Mol Microbiol. 1991 Feb;5(2):393–399. doi: 10.1111/j.1365-2958.1991.tb02121.x. [DOI] [PubMed] [Google Scholar]
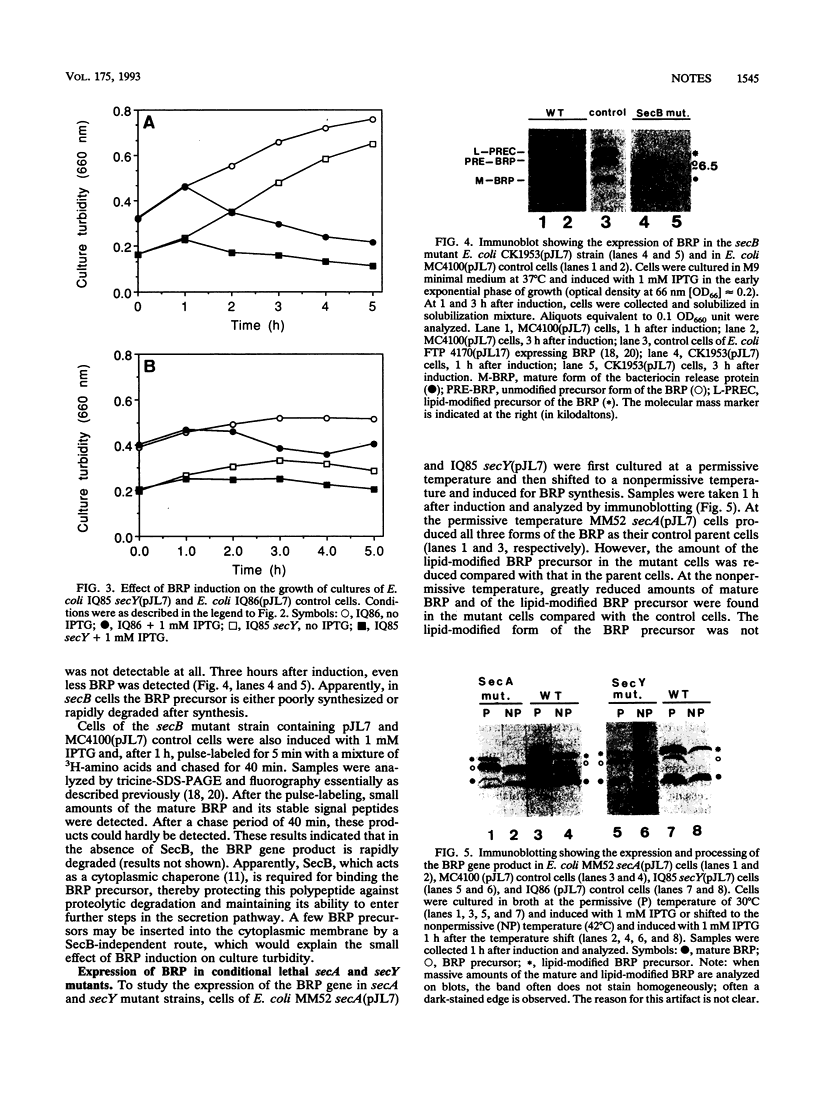
- Luirink J., Watanabe T., Wu H. C., Stegehuis F., de Graaf F. K., Oudega B. Modification, processing, and subcellular localization in Escherichia coli of the pCloDF13-encoded bacteriocin release protein fused to the mature portion of beta-lactamase. J Bacteriol. 1987 May;169(5):2245–2250. doi: 10.1128/jb.169.5.2245-2250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., de Graaf F. K., Oudega B. Uncoupling of synthesis and release of cloacin DF13 and its immunity protein by Escherichia coli. Mol Gen Genet. 1987 Jan;206(1):126–132. doi: 10.1007/BF00326547. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Cole S. T. An unmodified form of the ColE2 lysis protein, an envelope lipoprotein, retains reduced ability to promote colicin E2 release and lysis of producing cells. J Gen Microbiol. 1987 Sep;133(9):2411–2420. doi: 10.1099/00221287-133-9-2411. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Werner P. K., Müller M. Insertion of proteins into bacterial membranes: mechanism, characteristics, and comparisons with the eucaryotic process. Microbiol Rev. 1989 Sep;53(3):333–366. doi: 10.1128/mr.53.3.333-366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Driessen A. J., Hartl F. U., Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991 Mar 8;64(5):927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai M., Wu H. C. Export of the outer membrane lipoprotein is defective in secD, secE, and secF mutants of Escherichia coli. J Bacteriol. 1992 Apr;174(8):2511–2516. doi: 10.1128/jb.174.8.2511-2516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Hayashi S., Wu H. C. Synthesis and export of the outer membrane lipoprotein in Escherichia coli mutants defective in generalized protein export. J Bacteriol. 1988 Sep;170(9):4001–4007. doi: 10.1128/jb.170.9.4001-4007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Rice M., Wickner W. Effects of two sec genes on protein assembly into the plasma membrane of Escherichia coli. J Biol Chem. 1985 Feb 10;260(3):1836–1841. [PubMed] [Google Scholar]
- de Cock H., Overeem W., Tommassen J. Biogenesis of outer membrane protein PhoE of Escherichia coli. Evidence for multiple SecB-binding sites in the mature portion of the PhoE protein. J Mol Biol. 1992 Mar 20;224(2):369–379. doi: 10.1016/0022-2836(92)91001-6. [DOI] [PubMed] [Google Scholar]
- van der Wal F. J., Oudega B., Kater M. M., ten Hagen-Jongman C. M., de Graaf F. K., Luirink J. The stable BRP signal peptide causes lethality but is unable to provoke the translocation of cloacin DF13 across the cytoplasmic membrane of Escherichia coli. Mol Microbiol. 1992 Aug;6(16):2309–2318. doi: 10.1111/j.1365-2958.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]