Abstract
In a previous comparative proteomic study of Bacillus anthracis examining the influence of the virulence plasmids and of various growth conditions on the composition of the bacterial secretome, we identified 64 abundantly expressed proteins (T. Chitlaru, O. Gat, Y. Gozlan, N. Ariel, and A. Shafferman, J. Bacteriol. 188:3551-3571, 2006). Using a battery of sera from B. anthracis-infected animals, in the present study we demonstrated that 49 of these proteins are immunogenic. Thirty-eight B. anthracis immunogens are documented in this study for the first time. The relative immunogenicities of the 49 secreted proteins appear to span a >10,000-fold range. The proteins eliciting the highest humoral response in the course of infection include, in addition to the well-established immunogens protective antigen (PA), Sap, and EA1, GroEL (BA0267), AhpC (BA0345), MntA (BA3189), HtrA (BA3660), 2,3-cyclic nucleotide diesterase (BA4346), collagen adhesin (BAS5205), an alanine amidase (BA0898), and an endopeptidase (BA1952), as well as three proteins having unknown functions (BA0796, BA0799, and BA0307). Of these 14 highly potent secreted immunogens, 11 are known to be associated with virulence and pathogenicity in B. anthracis or in other bacterial pathogens. Combining the results reported here with the results of a similar study of the membranal proteome of B. anthracis (T. Chitlaru, N. Ariel, A. Zvi, M. Lion, B. Velan, A. Shafferman, and E. Elhanany, Proteomics 4:677-691, 2004) and the results obtained in a functional genomic search for immunogens (O. Gat, H. Grosfeld, N. Ariel, I. Inbar, G. Zaide, Y. Broder, A. Zvi, T. Chitlaru, Z. Altboum, D. Stein, S. Cohen, and A. Shafferman, Infect. Immun. 74:3987-4001, 2006), we generated a list of 84 in vivo-expressed immunogens for future evaluation for vaccine development, diagnostics, and/or therapeutic intervention. In a preliminary study, the efficacies of eight immunogens following DNA immunization of guinea pigs were compared to the efficacy of a PA DNA vaccine. All eight immunogens induced specific high antibody titers comparable to the titers elicited by PA; however, unlike PA, none of them provided protection against a lethal challenge (50 50% lethal doses) of virulent B. anthracis strain Vollum spores.
Bacillus anthracis, a gram-positive spore-forming bacterium, is the etiological agent of anthrax. Inhalation anthrax, the most severe form of the disease, is initiated by uptake of the infective spores by alveolar macrophages. The ingested spores germinate into vegetative bacilli which invade the bloodstream, where they multiply massively and express toxins and virulence factors. If not treated by prompt antibiotic administration, the disease results in death of the infected organism, as a consequence of toxemia and bacteremia. Due to the ease of infection by the respiratory route, the severity of the disease, and the ability of infective spores to survive harsh environmental conditions, B. anthracis is a major biothreat agent (9). Efforts to develop alternative or improved anthrax vaccination formulations have been expedited due to the accepted notions that existing anthrax vaccines only partially have the high protective potency expected for a long-lasting vaccine and the ability to provide immunization against multiple B. anthracis strains and/or meet the stringent safety requirements for large-scale human use (for reviews, see references 25, 52, and 60).
Virulent B. anthracis strains harbor two native plasmids, pXO1 and pXO2. The pXO1 plasmid carries the genes coding for the three components of the bacterial toxin which are essential for manifestation of the disease: lethal factor (LF), edema factor (EF), and protective antigen (PA). PA has no toxic effect by itself, yet it plays an essential role, recognizing and binding a membranal receptor on the surface of target cells (10, 12) and generating the portal which mediates the entry of EF and LF into the cells, where their detrimental activities occur. EF is a calmodulin-dependent adenylate cyclase that is responsible for the infection site-specific edema, and LF is a protease with a metal cofactor which targets host mitogen-activated protein kinases (44, 51, 53, 58, 73). The second virulence plasmid, pXO2, encodes proteins involved in the biosynthesis of the bacterial capsule, a poly-γ-d-glutamic acid immunologically inert entity involved in protection of the bacteria against phagocytosis. PA is a potent immunogen that is able to elicit a protective humoral immune response, and it is the basis for formulations of competent anthrax vaccines (25, 42, 48). A survey of the efficacy of PA for inducing protection against different B. anthracis isolates in several animal models showed that PA-mediated immunity varies significantly for B. anthracis virulent strains (21), suggesting that protective immunity may require additional bacterial factors. The existence of additional protective factors was also suggested by the observation that live attenuated B. anthracis strains (cured of the native virulence pXO1 and pXO2 plasmids) provide a certain level of immune protection (17), as do bacteria with a mutation in the pag gene (65). Administration of spores contributes to PA-mediated protective immunity (11), suggesting that some protective immunogens may be spore associated. Finally, it should be noted that live attenuated PA-producing anthrax vaccines (such as the pXO2− Sterne strain) exhibit significantly superior efficacy compared to PA preparations (25), yet such live vaccines, due to their high reactogenicities, are suitable only for veterinary purposes. Efforts to enhance the efficiency of protection conferred by PA by inclusion of LF, EF, or poly-γ-d-glutamate in preparations have been reported; conclusive evidence for improvement is yet to be obtained (25, 54, 59, 67, 72).
The search for bacterial antigens that are vaccine candidates by using a global proteomic approach relies on two selection criteria: protein localization and seroreactivity. The importance of protein localization stems from the notion that in the course of an infection, the bacterial outer cell membrane components, as well as secreted proteins, represent the interphase of the bacterium-host interaction and are exposed to the host immune system (14, 66, 75). To identify such proteins in the complex proteome of a pathogen, assessment of seroreactivity can be combined with proteomic studies for direct selection of proteins that are able to elicit a humoral immune response in the course of bacterial infections. This approach, in which large numbers of proteins separated by two-dimensional electrophoresis (2-DE) are identified by mass spectrometry and probed with immune sera (designated serological proteome analysis [SERPA] [43]), has been used with several bacterial systems for identification of diagnostic markers or vaccine candidates (6, 15, 20, 33, 35, 36, 46, 80, 82, 83) or for determination of specific circulating antibodies and/or disease markers in a variety of pathological states not related to diseases caused by bacteria (7, 43).
With the availability of the DNA sequences of the genomes of B. anthracis and related strains, we have embarked on a search for potential virulence-associated proteins and vaccine candidates among B. anthracis proteins using bioinformatic, proteomic, and large-scale serological global inspection techniques (5, 6, 15, 16, 28, 31). A preliminary limited proteomic and serologic study in which membranal proteins prepared from a nonvirulent B. anthracis strain were examined (6, 15) revealed several candidate immunodominant proteins. In a very recent report (16), we described an extended proteomic study in which virulent and nonvirulent strains of B. anthracis were subjected to a proteomic analysis which focused on the compositions of their secretomes under various growth conditions, including growth conditions that simulate the conditions encountered by the bacteria in the host. In the present proteomic serological analysis, we examined the reactivities of the secreted proteins with various immune sera collected from infected animals, and here we describe identification of about 50 proteins that exhibit different levels of immunogenicity. The present analysis and a previous analysis of membranal proteins (15), in combination with an analysis of more than 190 B. anthracis bioinformatically selected open reading frames (ORFs) (28), resulted in a list of more than 80 immunogens that are candidates for vaccine evaluation. Here we describe a pilot study in which we evaluated eight specific immunogens to determine their abilities to stimulate a humoral response and confer protection in a guinea pig model of anthrax following DNA immunization.
MATERIALS AND METHODS
Bacterial cultures and sample preparation for 2-DE.
The following B. anthracis strains were used in this study: fully virulent strain Vollum (pXO1+ pXO2+) and attenuated strains ΔVollum (pXO1− pXO2−) and Δ14185 (pXO1− pXO2−), a derivative of the nonproteolytic vaccine strain V770-NP1-R (= ATCC 14185) from which pXO1 was deleted (84). Cells were grown under aerobic conditions in FAG medium (17), in brain heart infusion (BHI) medium (Difco/Becton Dickinson, Maryland), or in NBY medium (0.8% [wt/vol] nutrient broth [Difco], 0.3% yeast extract [Difco], 0.5% glucose) for up to 24 h at 37°C with vigorous agitation or under semiaerobic conditions in NBY medium supplemented with 0.9% NaHCO3 in hermetically sealed filled flasks with slow agitation. Details concerning bacterial growth in the various media have been described previously (16). The low-nutrient (compared to FAG or BHI medium) NBY-CO2 medium promotes efficient toxin production (as detected by Western analysis) and capsule synthesis (which was visualized by negative staining using India ink [Becton Dickinson, Maryland]) and thus is considered to mimic infection conditions.
Secreted proteins were collected from the conditioned culture media essentially as described by Antelmann and coworkers (3). In brief, cultures were centrifuged to remove cells and filtered with 0.22-μm filters. One hundred milliliters of conditioned FAG or BHI medium or 200 ml of NBY medium was incubated at 4°C overnight in the presence of 10% trichloroacetic acid, and then the proteins were precipitated by centrifugation for 30 min in a Sorvall S34 (12,000 rpm). Pellets containing trichloroacetic acid-precipitated proteins were washed four times in a large volume of 96% ethanol and then resuspended by scrapping and extensive pipetting in 5 ml of an isoelectric focusing (IEF) sample solution composed of 8 M urea, 4% (wt/vol) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 40 mM Tris, 2% dithiothreitol (DTT), and 0.2 (wt/vol) Bio-Lyte 3/10 (Bio-Rad). The clear solution contained approximately 0.3 mg/ml protein.
2-DE separation of proteins and spot quantitation.
The secreted protein mixture (100 μg/run) was resolved first by IEF on pH 3 to 10 (nonlinear), ready-made, 17-cm immobilized pH gradient IPG strips (Immobiline DryStrips; Pharmacia) applied to a Protean IEF cell (Bio-Rad). IEF was carried out at 10,000 V for a total of 50,000 V·h, beginning with 250 V for 30 min. The strips were then processed for second-dimension separation by 10 min of incubation in a solution containing 6 M urea, 2% sodium dodecyl sulfate (SDS), 0.375 M Tris-HCl (pH 8.8), 20% glycerol, and 2% (wt/vol) DTT, followed by 10 min of incubation in a similar solution in which the DTT was replaced by 2% iodoacetamide. Strips were applied to 12.5% SDS-polyacrylamide gel electrophoresis (PAGE) gels, and electrophoresis was carried out with an Ettan DALT II system (Pharmacia). The gels were stained with Coomassie blue G-250 (Bio-safe Coomassie; Bio-Rad), and spots were detected and analyzed by scanning with a GS-800 calibrated densitometer and were quantified by using the PDQuest (Bio-Rad) or Image Gauge V4.0 (Fuji) software (see below). Analyses using the two computer applications generated similar results for individual protein abundance. In each case, at least two independently obtained secretomes representing the same biological sample were evaluated by 2-DE, and for each sample analyses with at least three gels were performed.
Identities of proteins and sequence analysis of B. anthracis ORFs.
The protein signatures and identities of the proteins in the secretomes investigated in this study have been described previously (16). Below, chromosomal ORFs are referred to by the NCBI locus tag identifiers for the B. anthracis Ames ancestor chromosome (69), and ORFs located on plasmids are referred to by their ORF identifiers as described by Okinaka and coworkers (61). The accession numbers for the Ames ancestor genome are NC007530 for the chromosome, NC007322 for pXO1, and NC007323 for pXO2 (www.ncbi.nlm.nih.gov/genomes/MICROBES/anthracis.html). Functional annotation of chromosomal or plasmid ORFs was performed as described by Ariel and coworkers (5, 6). Potential export signal peptides were identified by inspecting the N termini of the protein sequences for the presence of typical secretion signal N and H regions, as well as consensus cleavage sites (77, 78, 81), manually or using the SignalP (version 3.1) server (http://www.cbs.dtu.dk). The ligand specificities of transporters were determined by using the TransportDB database (http://www.memranetransport.org).
Hyperimmune and convalescent anti-B. anthracis antisera.
The sera used for two-dimensional Western analysis, which were similar to the sera described by Gat et al. (28), are described below. Hyperimmune antisera R-1, R-2, and R-3 were obtained from rabbits. R-1 was collected following multiple injections of live B. anthracis Sterne (pXO1+ pXO2−) spores (45), gradually increasing the dose from 105 to 109 spores per animal. R-2 was obtained after three injections of 106, 108, and 5 × 108 spores of a highly attenuated mutant strain of B. anthracis Vollum (pXO1+ pXO2+) generated by partial deletion of the pagA gene. R-3 was collected following multiple injections of 109 spores of the B. anthracis Δ14185 fully attenuated strain (pXO1− pXO2−). Antisera G-1, G-2, and G-3 were obtained using guinea pigs. G-1 was obtained after animals were exposed to a lethal challenge (104 spores) with the Vollum strain, followed by fluoroquinolone treatment, as described previously (2), and a rechallenge with the same dose of spores. G-2 was collected from animals vaccinated with 107 spores of an mntA highly attenuated mutant of the Vollum strain, as described previously (31). G-3 was collected 9 weeks after immunization of animals with 5 × 108 B. anthracis MASC spores, representing Δ14185 fully attenuated cells tailored to produce large quantities of recombinant PA. The sera were evaluated for the presence of antibodies against total vegetative bacterial antigens by an enzyme-linked immunosorbent assay (ELISA), using B. anthracis Δ14185 secreted and membrane proteins as the coating antigens (essentially as described by Aloni-Grinstein and coworkers [1]). Only sera (from individuals in the same treatment group) that elicited high antivegetative (secreted and membrane) bacterial antigen titers were pooled and used to probe Western blots. Anti-PA and anti-LF antibodies were detected by an ELISA as described previously (17, 30, 59).
Western blot analysis of 2-DE-separated proteins using anti-B. anthracis antisera.
For the Western blot analysis, 2-DE-separated proteins were electrotransferred onto Hybond nitrocellulose membranes (20 by 20 cm; Amersham Biosciences UK Ltd.) using a Hoefer-DALT Western vertical buffer circulating chamber (Pharmacia) in Tris-glycine buffer (25 mM Tris, 192 mM glycine, 20% methanol; pH 8) for 16 to 20 h with a constant current of 230 mA. Western blots were probed with the anti-B. anthracis sera at a dilution of 1:1,000 (unless indicated otherwise) and were developed by the enhanced chemiluminescence (ECL) method with horseradish peroxidase-conjugated secondary antibodies (diluted 1:5,000; Amersham Biosciences), and this was followed by horseradish peroxidase detection using the SuperSignal West Pico ECL substrate (Pierce).
Quantification of immunoreactivity.
Determination of the immunogenicity score (IS) of a given protein was based on SERPA (15), where the intensity of the protein spot on a Coomassie blue-stained gel (which reflected the abundance of the protein) was compared to the intensity of the signal generated by the corresponding protein spot on the Western blot (which represented the immunoreactivity of the protein with the sera used). A partial overview of the SERPA is shown in Fig. S1 in the supplemental material. The intensities of the protein spots on Coomassie blue-stained gels (abundance) were determined as described by Chitlaru et al. (16), except that the scanning data were analyzed using the Image Gauge (Fuji) software, which was also used to quantify the intensities of the signals generated by the protein spots on Western blots. The luminescence emitted by ECL-developed 2-DE Western blots was measured using a LAS-3000 luminescence scanner (Fuji), which allowed real-time monitoring of luminescence signals over a linear intensity range of 4 orders of magnitude. (Upon quantification, the signals generated quantity level values, which reflected the luminescence emission and light source density on a blot.) At least five images obtained with different exposure times were inspected for each blot. The images selected for signal quantification were obtained with exposure times with which most of the strong luminescence signals were not saturated. In some cases, for strong immunogens for which saturation of the signal could not be avoided (such as some occurrences of PA), the value obtained (see below) was thought to be an underestimate of the actual IS. Alternatively, 2-DE signatures of secretomes in which a particular strong immunogen was less abundant were inspected. For normalization of all individual Coomassie blue-stained spots (abundance of individual spot/sum of abundance values for all spots) under all growth conditions examined, we used reference protein spots with essentially identical intensities in various secretomes (16). For a particular protein, the IS was calculated using the equation IS = quantity level of individual spot/(abundance of individual spot/sum of abundance values for all spots). When images obtained with different exposure times were compared to the same Coomassie blue-stained gel, the ISs were normalized using an internal calibration curve generated by spotting different amounts of an ECL-developed peroxidase-conjugated antibody control. To validate the ISs, we used as an “internal standard” the S-layer protein Sap, which was seroreactive with all antisera tested and was present in all B. anthracis secretome 2-DE maps inspected except those generated with the NBY-O2 medium (16).
For computation of all ISs, the data for at least three independent pairs of Coomassie blue-stained gels and matching 2-DE blots were averaged. In all cases, the IS for a particular protein with a particular serum was highly reproducible (less than 30% deviation). Immunoreactivity signals which could not be attributed without ambiguity to a particular protein on the matching 2-DE map were not considered.
Construction of eukaryotic expression plasmids, DNA immunization with plasmid DNA, and measurement of the immune response.
The DNA immunization procedure was used for evaluation of the immunogenic potentials of selected ORF products. Individual ORFs were cloned in the eukaryotic expression vector pCI (Promega), which carries the eukaryotic cytomegalovirus promoter, a recombinant chimeric intron, and the simian virus 40 polyadenylation signal for efficient expression in mammalian cells, in addition to the T7 promoter for in vitro transcription and translation (T&T) expression (29, 34). The plasmid DNA used for gene gun immunization was prepared by an alkaline lysis method, followed by CsCl gradient centrifugation. The purified DNA preparations were solubilized in pyrogen-free water and kept frozen. The immunization protocols used were essentially the protocols described previously (29, 34). For gene gun vaccination (Helios gene gun system; Bio-Rad), plasmid DNA was precipitated onto 1-μm-diameter gold particles at a ratio of 2 μg per mg of gold and loaded onto Gold-Coat tubing (Bio-Rad) using polyvinylpyrrolidone as an adhesive. Gene gun shots (0.5 μg DNA) were directed onto exposed abdominal dermis, and the protocol included three or four immunizations of guinea pigs (250- to 300-g females; Charles River Laboratories, Margate, United Kingdom) at 2-week intervals. Each DNA vaccination group included 10 experimental animals. Animals were bled for serum collection by cardiac puncture. The immune responses elicited in the animals following DNA immunization were determined by an ELISA for animals immunized with DNA coding for PA and by quantitative immunoprecipitation (IP) titration for animals immunized with the other ORFs investigated (34). The latter method is based on IP titration of 35S-labeled T&T products (usually 1 to 2 μl of the reaction mixture) with serial dilutions of the antiserum (in a final volume of 100 μl). The immunoprecipitated proteins were analyzed by both SDS-PAGE and autoradiography and were quantified using a β-counter (1600 TR liquid scintillation analyzer; Packard). The final dilution that allowed detection of the immunoprecipitated T&T polypeptide by SDS-PAGE or the dilution that exhibited a measurable level of radioactivity (in cpm) which was at least three times the level of radioactivity of the background was considered to represent the specific IP titer of a serum. In all cases, the titers determined by the two procedures were identical.
Infection challenge of guinea pigs.
Immunized animals were infected with 50 50% lethal doses (LD50) of Vollum spore preparations administered subcutaneously (LD50, 100 spores). Prior to infection of the animals, the spore preparations were heat shocked (70°C, 20 min) to synchronize germination. The animals were observed daily for 21 days.
Care of experimental animals.
Animals were handled in accordance with the National Research Council 1996 Guide for the Care and Use of Laboratory Animals according to protocols approved by the Animal Use Committee of the Israel Institute for Biological Research.
RESULTS
Identification of B. anthracis seroreactive proteins and determination of their relative immunogenicities.
Recently, we determined the proteomic 2-DE maps of secretomes generated by wild-type and plasmid-cured strains of B. anthracis under various growth conditions (16). Western blots derived from the different 2-DE secretome gels were probed with various anti-B. anthracis specific hyperimmune sera (see Materials and Methods for a description of the sera; see Fig. S1 in the supplemental material for a partial overview of the analysis). Comparison of the protein reference 2-DE signatures with the corresponding Western immunoblots allowed us to identify the immunogens (Fig. 1) and to quantitatively evaluate their immunogenicities (Fig. 2) (see below). The limited nature of the proteomic approach (which relied on expression of the proteins under the particular set of physiological conditions used for generation of the biological samples investigated) imposed severe restrictions on the repertoire of proteins detected. Yet, by using a battery of six distinct rabbit and guinea pig antisera, 49 immunogenic proteins were detected in the secretomes of B. anthracis generated under different growth conditions (Table 1). Eleven of these immunogenic secreted proteins were also detected in a cell-associated form in previous studies (5, 15, 31). Thus, this study revealed 38 new immunogenic polypeptides. Together, the results of SERPA of secreted proteins in this study and of membranal proteins in previous studies allowed us to identify 58 immunogenic proteins (including PA, EF, and LF) (Table 1). Thus, 66% of the 88 ORFs encoding the most abundant exposed proteins under various conditions were immunogenic.
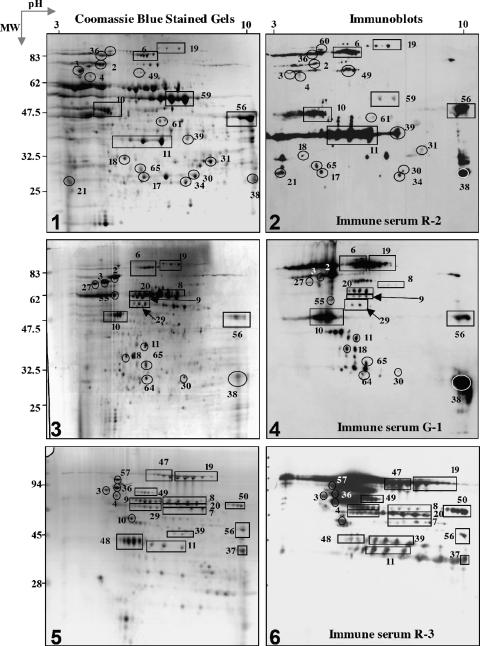
FIG. 1.
SERPA of B. anthracis secreted proteins: comparison of Coomassie blue-stained 2-DE (gels 1, 3, and 5) with the corresponding Western blots (gels 2, 4, and 6). (Gels 1 and 2) FAG medium secretome of the Vollum strain. (Gels 3 and 4) BHI medium secretome of the Vollum strain. (Gels 5 and 6) FAG medium secretome of the Δ14185 strain. Blots were probed with the sera indicated (see Materials and Methods for a description of the sera). See Table 1 for the identities of the marked protein spots and a complete list of immunogenic proteins. The protein spot numbering is the numbering on the proteomic maps reported previously (16). Also see Fig. S1 in the supplemental material for an extended overview. MW, molecular weight.
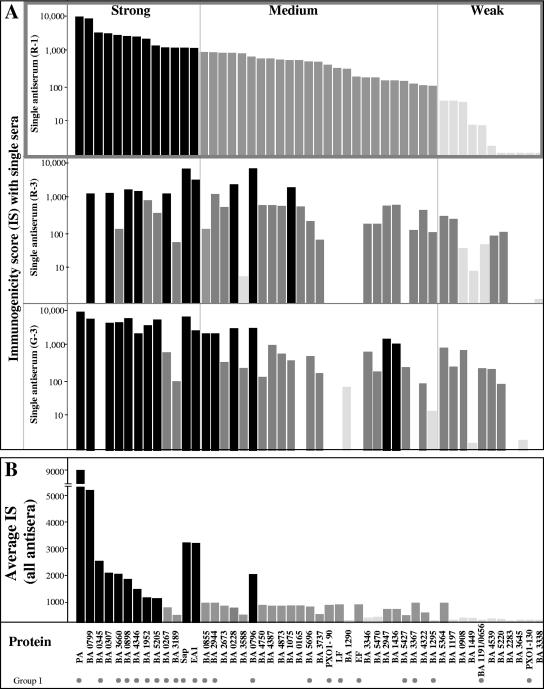
FIG. 2.
ISs of B. anthracis secreted proteins. The immunogenicities of proteins identified by SERPA were determined by quantitative comparison of the levels of the various proteins with the intensities of the corresponding Western signals, as described in Materials and Methods. (A) ISs calculated from Western blots probed with rabbit antisera R-1 and R-3 and guinea pig antiserum G-3. (B) Average ISs calculated by probing with all six antisera used in this study. Proteins are arranged in descending order according to their R-1 ISs and are categorized arbitrarily as strong immunogens (IS, > 1,000) (solid bars), medium immunogens (1,000 > IS > 100) (dark gray bars), and weak immunogens (IS, <100) (light gray bars). Immunogens which were also detected by IP assays of in vitro products, as detected in the genomic serologic screen described by Gat et al. (28, 29), are indicated by dots at the bottom and, by definition, belong to group I described in Fig. 3.
TABLE 1.
Immunogenic B. anthracis proteins identified by SERPA
| Accession no.a | Proteinb | Domain(s)c | Expression under high-CO2 conditionsd | Membranal fractione | 2-DE spotf | Confirmed by IPg | Virulence relatedh |
|---|---|---|---|---|---|---|---|
| pXO1-90 | Unknown | S, SLH | + | 51 | + | ||
| pXO1-107 | LF | S | + | 52 | + | + | |
| pXO1-110 | PA | S | + | 6 | + | + | |
| pXO1-122 | EF | S | + | 60 | + | + | |
| pXO1-130 | SBP-ABC [Zn] (AdcA) | S | + | 73 | + | ||
| BA0108 | Elongation factor (EF-Tu) | S | + | ||||
| BA0165 | Prolyloligopeptidase | S | 36 | + | |||
| BA0228 | ATP-ABC (YdiF) | + | 23 | ||||
| BA0267 | Heat shock protein (GroEL) | + | + | 4 | + | + | |
| BA0307 | Unknown lipoprotein (YerH) | S | + | 61 | |||
| BA0309 | δ-1-Pyrroline-5-carboxylase (RocA) | + | + | ||||
| BA0345 | Hydroperoxide reductase (AhpC) | + | 67 | + | + | ||
| BA0656 | SBP-ABC [oligopeptide] | S | + | 8 | + | + | |
| BA0796 | Unknown | S, SH3, 3D | + | 56 | + | ||
| BA0799 | Unknown | S, HlyD | + | 39 | |||
| BA0855 | SBP-ABC [amino acid] (YckK) | S | + | 30 | + | ||
| BA0885 | S-layer protein (Sap) | S, SLH | + | + | 19 | + | |
| BA0887 | S-layer protein (EA1) | S, SLH | + | + | 47 | + | |
| BA0898 | Alanine amidase III (CwlB) | S, SLH | + | + | 50 | + | + |
| BA0908 | SBP-ABC [oligopeptide] | S | 28 | + | |||
| BA1075 | Nuclease/phosphatase | S | 54 | ||||
| BA1129 | Unknown | S, SLH | + | ||||
| BA1191 | SBP-ABC [oligopeptide] | S | + | 8 | + | + | |
| BA1197 | SBP-ABC [oligopeptide] | S | + | 20 | + | ||
| BA1290 | Camelysin (TasA) | S | + | 21 | + | ||
| BA1295 | Metalloprotease (InhA1) | S | + | 2 | + | + | |
| BA1436 | Sulfatase (YvgJ) | S | 53 | + | |||
| BA1449 | Peptidase M23/M37 | S | 42 | ||||
| BA1818 | Alanine amidase IV | S, SLH | + | + | |||
| BA1952 | NlpC/P60 endopeptidase | S, NlpC/P60, SH3 | + | 38 | + | + | |
| BA2283 | Unknown | 55 | |||||
| BA2346 | Methylcitrate dehydratase (MmgE) | + | |||||
| BA2673 | Chitosanase | 62 | |||||
| BA2944 | Polysaccharide deacetylase (YjeA) | S | + | 34 | + | + | |
| BA2947 | Sulfatase (YflE) | S | 29 | + | |||
| BA3189 | SBP-ABC [Mn] (MntA) | S | + | + | 18 | + | + |
| BA3338 | Unknown | S, SLH | + | 48 | |||
| BA3346 | 6-Aminohexanoate dimer hydrolase | S | 33 | ||||
| BA3367 | γ-Phage receptor (GamR) | S, LPXTG | + | 3 | + | ||
| BA3588 | Lipoprotein (VanW) | S | + | 17 | |||
| BA3609 | Aldehyde dehydrogenase (DhaS) | + | |||||
| BA3645 | SBP-ABC [oligopeptide] | S | + | 69 | + | ||
| BA3660 | Serine protease (HtrA) | S | + | 11 | + | + | |
| BA3737 | Alanine amidase II (CwlA) | S, SLH | + | + | 49 | + | + |
| BA4322 | Nucleotidase | S | + | 9 | + | ||
| BA4346 | 2,3-Cyclic nucleotide diesterase (YfkN) | S, LPXTG | + | + | 57 | + | + |
| BA4387 | Leucine dehydrogenase (LeuDH) | 70 | |||||
| BA4539 | Heat shock protein (DnaK) | + | + | 1 | + | ||
| BA4705 | Peptidyl-prolyl isomerase (RopA) | + | |||||
| BA4750 | Carboxypeptidase (VanY) | S | 31 | ||||
| BA4873 | Alanine dehydrogenase (Ald-2) | 71 | |||||
| BA5220 | SBP-ABC [methionine] | S | 65 | ||||
| BA5364 | Enolase (Eno) | + | 5 | + | |||
| BA5427 | Endopeptidase (LytE) | S, NlpC/P60 | + | 59 | + | + | |
| BA5470 | Sulfatase (YvgJ) | S | + | 10 | + | ||
| BA5576 | Fructose biphosphatase (GlpX) | + | |||||
| BA5696 | Superoxide dismutase (SodA-2) | + | 64 | + | |||
| BAS*5205 | Collagen adhesion protein | S | 27 | + | + |
Seroreactive proteins identified by serological proteome analysis are listed in ascending order of the accession number (TIGR identification tag).
The designations for some of the proteins are in parentheses. The putative functional annotation of the proteins is the annotation of Ariel et al. (5, 6). The SBPs of ABC transporters are indicated by SBP-ABC, and their inferred ligand specificities (according to the TransportDB database) are indicated in brackets.
S, export signal; SLH, S-layer homology domain; NlpC/P60, cell wall peptidase family domain; SH3, Srk homology domain; HlyD, involved in secretion of virulent factors in gram-positive pathogens; 3D, cation binding domain involved in protein-protein interaction.
Proteins expressed under high-CO2 conditions, as determined previously (16), are indicated by plus signs.
The table includes 20 immunogens which were identified by SERPA of B. anthracis membranal enriched fractions (6, 15); 14 of these immunogens were also identified in the bacterial secretome and therefore were assigned a spot number (16). The proteins identified only in the membranal fraction are BA0108, BA0309, BA1129, BA1818, BA2346, BA3609, BA4705, and BA5576.
The numbers are the numbers assigned to the protein spots on the 2-DE maps of the secretome, as described previously (16). Some of the immunogenic protein spots are present on the gels and Western blots shown in Fig. 1.
The immunogenicities of the proteins indicated by plus signs were also determined by quantitative IP of in vitro translation products with immune sera in the context of the screen described by Gat et al. (28) (group I proteins in Fig. 3).
Orthologs of proteins involved in virulence and pathogenicity, as described previously (16), are indicated by plus signs.
The vast majority of the immunogens identified by SERPA are proteins that have putative biological functions or extracellular localization domains. Only one protein, BA2283, lacks any recognizable domain. A prevalent category of immunogenic proteins includes the proteins exhibiting S-layer homology (SLH) domains. In this study we identified eight immunogenic SLH proteins, including Sap (BA0885) and EA1 (BA0887), as well as pXO1-90, BA0898, BA1129, BA1818, BA3338, and BA3737. In a recent genomic serological screen based on in vitro expression of selected genes (28), three additional SLH proteins (pXO1-54, pXO2-42, and BA0981) were identified. Therefore, a total of 11 SLH proteins have been identified as immunogens, representing 50% of the SLH proteins encoded in the genomes of the bacteria. One other outstanding functional group of immunogenic polypeptides is the group containing the solute binding subunits (SBPs) of ABC transporters; eight new immunogenic proteins (BA1191, BA0656, BA0855, BA1197, BA0908, BA5220, BA3645, and pXO1-130) were identified in addition to the six previously described immunogenic SBPs of ABC transporters (28).
Rank order of immunogenicities.
In order to evaluate in a somewhat more quantitative manner the relative potencies of the various immunogens and to attempt to prioritize the immunogens for further study (see Materials and Methods), we determined the intensities of the signals generated on the Western blots and normalized these intensities to their relative intensities on the Coomassie blue-stained gels (protein abundance). The ratio of the immunoblot signal to the protein abundance is referred to as the IS of a protein. The scores for all immunogens are summarized in Fig. 2. Proteins exhibited a wide dynamic range of ISs with respect to their reactivities with a particular serum, and differences of up to 4 orders of magnitude were calculated for the different proteins present on the same proteomic map, as shown by the ISs obtained with individual sera R-1, R-3, and G-3 (Fig. 2A). Serum R-1, which allowed us to visualize all immunogens (Fig. 2A), was used for preliminary categorization of proteins as strong immunogens (IS, >1,000), medium immunogens (1,000 > IS > 100), and weak immunogens (IS, <100). Diverse ISs could be obtained with the various antisera, yet the mean immunogenicity calculated by averaging the ISs obtained using the various sera appeared to largely parallel the immunogenicity obtained with the R-1 serum and did not significantly alter the distribution of the proteins in the three IS classes. For example, the reactivities with the R-1 antiserum showed that only 2 (BA0267 and BA3187) of the 12 proteins which belong to the strong IS class (ISs, 1,300 and 1,000, respectively) were classified as medium immunogens when the averages for all sera were used (ISs, 800 and 500, respectively); furthermore, BA0796 was the only immunogen in the medium immunogen group detected with the R-1 serum (IS, 800) which was classified as a strong immunogen (IS, 2,500) when the averages for all sera were used (Fig. 2).
As expected, the strongest immunogen was PA (Fig. 2; see Fig. S1 in the supplemental material [Western blots with sera R-1 and G-1, in which most of the luminescence signal was contributed by PA]). The other constituents of the toxins (LF and EF) are relatively moderate immunogens and belong to the medium immunogen group (Fig. 2), in line with previous studies in which their immunogenicities were examined (65). In addition to PA and the S-layer proteins Sap and EA1, which are known to be highly immunogenic, the strong immunogens (Fig. 2) include the following proteins: the SBP of the Mn ABC transporter MntA (BA3189), the chaperonin and heat shock protein GroEL (BA0267), the peroxidase AhpC (BA0345), an SLH protein having alanine amidase activity (BA0898), a 2,3-cyclic nucleotide diesterase (BA4346; a homolog of the YfkN protein of Bacillus subtilis), the protease HtrA (BA3660), a protease belonging to the NplC/P60 family (BA1952), and a collagen adhesin (BAS5205). All of these immunogens are either B. anthracis virulence factors (e.g., MntA [31]) or homologs of proteins involved in the virulence of other pathogenic bacteria. For example, although GroEL is considered a housekeeping protein, it was shown to be active in the virulence of Chlamydia, Listeria, and Salmonella (71, 26, 12). AhpC is required for survival of Legionella pneumophila and Mycobacterium leprae in macrophages (68, 64) and is immunogenic in Salmonella enterica (76), Mycobacterium tuberculosis (74), and Helicobacter pylori (62). AhpC also was identified as the strongest immunogen by SERPA of B. anthracis membranal proteins (15). Alanine amidases, such as BA0898, have been determined to be involved in the virulence of a variety of pathogens, including S. enterica (23) and Listeria monocytogenes. 2,3-Cyclic nucleotide diesterase is involved in the virulence of Clostridium perfringens (8) and Yersinia enterocolitica (85). Finally, the chaperone HtrA, which is part of the complex responsible for the Sec secretion pathway in bacilli, is the major CO2-induced protease in B. anthracis (16) and is considered a virulence factor and vaccine candidate in many pathogens (18, 35, 40, 41, 55, 57). The following three proteins having unknown functions were identified as strong immunogens: BA0307 (a lypoprotein homologous to YerH of B. subtilis) and BA0796 and BA0799 encoded by genes located close to each other in the chromosome. Both of the latter proteins have virulence-related domains; BA0799 has a partial HlyD domain that is involved in ABC transporter-mediated type I secretion of virulence factors in gram-negative pathogens (50), and BA0796 has an SH3 (src homology) eukaryote-type virulence-related domain, as well as a three-dimensional domain for cation binding. BA07 99 is the second strongest immunogen identified in this study. It is interesting that, consistent with their high immunogenicities, 7 of the 14 strong immunogens have also been detected in a membranal form (Table 1) (15) and 9 of them were detected in the B. anthracis secretome obtained from cultures grown in the low-nutrient bicarbonate-supplemented NBY-CO2 medium (Table 1) (16), conditions which are thought to mimic those encountered in the host during infection.
Evaluation of the protective values of selected immunogenic proteins by DNA-mediated immunization of guinea pigs.
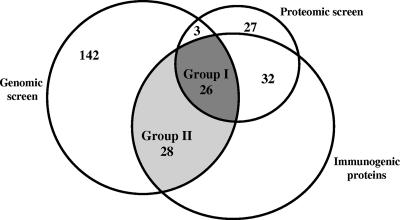
Of the 58 B. anthracis immunogenic proteins identified by proteomic analysis (Table 1), 26 were also determined to be immunogenic by a parallel genomic screen (28) (group I in Fig. 3). The genomic screen identified 28 ORFs (group II) (Fig. 3) in addition to these 26 immunogens detected in the present study. An initial pilot study included, in addition to the control potent immunogen PA (a strong immunogen) (Fig. 2), two strong immunogens belonging to group II (BA2805 and BA4787) and two medium immunogens, one belonging to group I (BA3367) and one belonging to group II (BA0672), as well as one pXO1-encoded immunogen belonging to group I (pXO1-130) and one immunogen belonging to group II (pXO1-54). Selection was guided not only by the relative immunogenic strengths of the various proteins but also by additional criteria, such as potential involvement in virulence, as is the case for BA0345/AhpC and BA3189/MntA (which are described above) or BA0673/InhA, BA4787, and BA2805, which were proposed recently (28) to have a possible role in virulence. The proteins selected included proteins having export signals, as well as membrane anchorage motifs (LPXTG, SLH, or a lipobox) (Fig. 4), such as most of the proteins mentioned above, as well as BA3367/GamR (a protein having an unknown physiological role that is a γ-phage receptor [19]), pXO1-54, and pXO1-130. The latter protein encodes a putative SBP of an ABC transporter specific for Zn (AdcA) and therefore is potentially associated with functions requiring Zn as a cofactor, such as the proteolytic activity of the toxinogenic factor LF. We also noted that pXO1-130 was detected in media that mimicked in vivo conditions with high CO2 concentrations in previous proteomic analyses (16, 49).
FIG. 3.
Segregation of immunogenic proteins identified by SERPA and by the genomic-serologic screens into various groups. The genomic screen (left circle) of 199 ORFs (28) allowed identification of 54 immunogenic proteins (group I and group II). The combined proteomic screens described in the present study and by previous SERPA (6, 15) allowed identification of 88 proteins (small circle), 58 of which are immunogenic proteins and 26 of which are included in group I.
FIG. 4.
Humoral immune responses and protective values of selected ORF products as assessed by DNA-mediated immunization. (Upper left panel) Description of the immunogenic proteins selected for analysis. S, export signal. (Upper middle panel) Immunogenicities of the proteins as determined by SERPA and/or by IP of their 35S-radiolabeled T&T products (see Materials and Methods). (Upper right panel) Specific antibody responses following DNA immunization of guinea pigs by gene gun vaccination using the administration regimen described in Materials an Methods. Immune responses were determined quantitatively by IP of tertiary serial serum dilutions of 35S-labeled translation products. (Lower panel) Survival of guinea pigs (10 guinea pigs per group) and mean times to death (MTD) following a challenge with 50 LD50 of fully virulent spores of the B. anthracis Vollum strain injected subcutaneously.
Guinea pigs were immunized with plasmids carrying the genes encoding the immunogenic proteins described above. Every group of animals was immunized four times with DNA encoding one of the immunogens. In the absence of an appropriate ELISA, the induction of specific humoral immune responses was evaluated by performing quantitative IP assays for 35S-labeled in vitro products (29). Immunization with plasmid DNA encoding each of the immunogens resulted in a specific immune response against the protein at a level commensurate with the level obtained after immunization with DNA encoding PA (Fig. 4). Interestingly, the highest antibody titers (1:36,000) were obtained only after immunization with the strong immunogens (BA3189, BA2805, and PA); the lowest titer was obtained with the weak immunogen pXO1-130, yet one of the strong immunogens (BA0345) induced a similar low antibody titer. These observations indicate that the immunogens used may elicit immune responses when they are administered individually and not only in the context of infection. Immunized animals were challenged with a lethal dose (50 LD50) of the B. anthracis Vollum strain. Except for one animal, all guinea pigs immunized with PA survived the challenge. On the other hand, all animals immunized with the DNA encoding any of the other immunogens died after the challenge. Some experimental groups exhibited a slightly extended mean time to death compared to animals that were mock immunized with only the expression plasmid, yet the differences were not statistically significant.
DISCUSSION
There is very limited information concerning B. anthracis in vivo-expressed pathogenicity-related genes other than the genes coding for the bacterial toxins LT and ET and for the biosynthesis of the antiphagocytic capsule. The S-layer proteins have been found to be expressed during infection, based on the massive presence of anti-S-layer antibodies in infected animals (24). In addition, in vivo expression of several individual genes or operons has been suggested on the basis of the observation that disruption of these genes influences the virulence of the bacteria; in a murine model of infection in which the attenuated Sterne strain was used, it was shown that the LD50 and/or the mean time to death were altered by disruption of three phospholipases C encoded by the plcB, smcA, and plcA loci (37), a cell-wall d-alanine esterification system encoded by the dltABCD operon (22), the siderophore anthrachelin encoded by the asbA operon (13), and possibly two of the four B. anthracis superoxide dismutases encoded by the sod-15 and sodA1 loci (63). Recently, the in vivo expression of the cholesterol-dependent cytolysin anthrolysin O was revealed by reverse transcription-PCR analysis of spleens from B. anthracis-infected mice (70). Finally, we have shown that MntA, an ABC transporter involved in the import of Mn, is a major virulence determinant in B. anthracis (31). Recently, we described a proteomic study of B. anthracis in which the influence of the virulence plasmids on the secretome composition under various growth conditions was examined (16). This study generated a map consisting of 400 protein spots, which allowed identification of the 64 most abundant expressed proteins, many of which are established virulence factors in other pathogens. Here we describe a subsequent analysis of these proteins, performed using a battery of sera from B. anthracis-infected animals, which allowed identification of the proteins which are both expressed in infected animals and immunogenic. Of the 64 proteins, 49 were found to react with the sera. The immunogenic proteins identified belong to all of the previously defined categories of the B. anthracis secretome, including toxins, enzymes, ABC transporters, protein folding mediators, and stress/detoxification proteins (for details, see references 4, 32, 49, and 16). We recently attributed possible virulence/pathogenicity-associated functions to at least 33 of the 64 secreted proteins, based on their orthologs in other pathogenic bacteria (16). One of the most striking observations in the current study was that almost 90% of these 33 proteins (29 immunogens [Table 1]) were expressed following infection of either guinea pigs or rabbits with B. anthracis.
On the basis of the results of this proteomic study and the results generated by a similar SERPA performed with a membranal proteome (15) of B. anthracis, it appears that 58 of 88 proteins identified are immunogenic (Table 1 and Fig. 3). This high frequency of immunogenic proteins (at least 78% of the most abundant proteins of the bacterial secretome and 64% of the prevalent membranal proteins) is consistent with the generally accepted notion that secreted and membranal proteins are preferential targets in the search for vaccine candidates.
A large-scale genomic search for B. anthracis immunogens (28) based on probing the reactivities of in vitro-generated polypeptide products of almost 200 selected ORFs with the same set of anti-B. anthracis antisera identified 54 immunogenic proteins. Almost 50% of these proteins (26 gene products) were also identified by the SERPA (group I) (Fig. 3). This very high percentage is quite remarkable, since the genomic screen relied only on predictions that ORFs were either secreted or membrane associated and there was no information concerning their abilities to be expressed in the bacteria either in vitro or in vivo (this was unlike the SERPA, in which only the abundant proteins could be analyzed). Moreover, almost 90% of the 29 proteins identified by both the genomic screen and the proteomic screen belong to group I of the immunogenic proteins, and no less significantly, almost all strong immunogens (12 of 14 immunogens) identified in this study (Fig. 2) also belong to group I (Fig. 3). The practical significance of these observations is that the two serological screens, the screen based on inspection of in vitro-generated ORF products and the screen based on SERPA, are complementary and should be used for defining a comprehensive list of vaccine candidates. The two screens contributed to the pool of 86 immunogens identified almost equal numbers of proteins, and the overlapping group comprised about one-half of the immunogens identified (Fig. 3). To the best of our knowledge, this is the most extensive list of this type of in vivo-expressed B. anthracis proteins published to date.
Two classes of proteins were identified as highly immunogenic in the present proteomic study, the class consisting of SBPs of ABC transporters and the class containing SLH domains. These two classes are both characterized by the abilities of their members to have dual membrane and secretome localizations. According to the genomic database, 55 recognized SBPs of ABC transporters are encoded in the genome of B. anthracis; therefore, the 16 immunogenic ABC transporters detected by the serologic analysis represent 25% of all of the ABC transporters encoded. This is a very high proportion, considering the limitations of the screen, and it strongly supports the concept that ABC transporters represent a preferred category of proteins for vaccine development (27). As explained above, one of the SBPs of ABC transporters, MntA, was recently shown to be a novel virulence factor of B. anthracis (31). It is worth noting that BA0799, the most immunogenic protein (besides PA) determined by our analysis (Fig. 2), may also be a member of the ABC transporter group; although BA0799 is still categorized as a protein having an unknown function, it has a HlyD domain present during secretion of virulence factors in gram-negative bacteria (50). The presence of this domain, together with the genomic localization of BA0799 adjacent to two genes encoding ABC transporter subunits (BA0797 [the ATP binding subunit] and BA0798 [the membrane subunit]), indicates that this potent immunogen may also be a component of an efflux ABC transporter. The other prevalent category of immunogenic proteins is the category containing the proteins having SLH domains. Eleven SLH proteins were found to be immunogenic by the serological screens. This represents 50% of the 22 SLH proteins encoded in the genome of B. anthracis. Sap and EA1, the proteins constituting the S-layer, have been found to be immunogenic (24), yet in vivo expression of the other proteins containing SLH domains, which are not part of the S-layer, has not been described. We documented previously that SLH domains are a prevalent membrane docking modality in B. anthracis (15), and many SLH proteins, other than Sap and EA1, reside both on the bacterial surface and in the secretome (16). Given the localization, immunogenicity, and abundance of the SLH proteins in B. anthracis, they may still be a class of targets that are useful for forensics and/or diagnostic purposes.
In two additional studies workers have recently examined the immunome of B. anthracis in search of potential protective immunogenic proteins. Kudva and coworkers (47), by using an expression library enriched in clones representing ORFs encoding spore-associated proteins, determined the immune reactivities of these proteins against human sera derived from individuals vaccinated with anthrax vaccine adsorbed. These workers established the presence of some spore proteins in the purified PA preparation used for immunization and their immunogenicities, yet these immunogens are irrelevant for the expression of proteins in vivo and there is no evidence that protection induced by immunization with PA-derived subunit vaccines can be attributed to any protein present in the vaccine other than PA. In a second study, DelVecchio et al. (20) examined the presence of anti-spore protein antibodies in human sera collected from convalescent patients with cutaneous anthrax by performing SERPA of spore extracts. In this study the workers distinguished 15 immunogens, about one-half of which were not identified in our serological survey. These immunogens include alanine recemase and CotJc, which are known spore constituents (38, 56, 79), several enzymes involved in ATP generation which may be relevant to the germination process and therefore particularly abundant in spores, such as glyceraldehyde-3-phosphate dehydrogenase, and three subunits of the pyruvate dehydrogenase complex (39, 56). Of particular interest is the observation, reported by DelVecchio and coworkers (20), that the neutral protease NprB (BA0599) is immunogenic. In our study, NprB did not react with any sera tested, despite its overwhelming abundance (80% of the aerobic low-nutrient medium [NBY] secretome). Furthermore, the complete lack of immune reactogenicity was consistent with its dramatic downregulation (essentially complete shutoff) in cells grown in the same low-nutrient medium supplemented with CO2 (16), conditions which are known to induce synthesis of LT and ET and are considered to mimic the conditions encountered by the bacteria in the infected host. Furthermore, the shutoff of NprB in vivo was consistent with the observation that its proteolytic activity may be deleterious to the integrity of PA (16). It is tempting to speculate that the difference between the results of the two studies with respect to NprB immunogenicity resulted from the fact that the sera used by DelVecchio et al. were collected from convalescent cutaneous anthrax patients and therefore the reported in vivo expression of NprB may be associated with cutaneous anthrax.
We are now involved in a comprehensive study attempting to evaluate the potentials of the 84 immunogenic proteins to provide protective immunity. For an initial pilot study we selected immunogens representing group I and group II, including both chromosome- and pXO1-encoded proteins. We were disappointed that none of these immunogens provided protective immunity (Fig. 4) or even resulted in a significant increase in the mean time to death. Notably, most of the strong immunogens were also the most potent immunogens for inducing humoral responses when they were administered as DNA vaccine and in this respect were as potent as PA. While one could argue that a DNA vaccine is not necessarily the best route of immunization for evaluation of the immunogenicity or efficacy of a given bacterial polypeptide, it is quite evident that when this route of administration is used, the selected immunogens cannot be compared with the very efficacious PA DNA vaccine which was used as a positive control. Although we cannot rule out the possibility that the immune responses elicited individually by all eight proteins tested are not protective, it is possible that by using different modes of vaccination with the purified proteins (alone or in combination with other B. anthracis immunogens), using appropriate adjuvants, by exploiting a live attenuated B. anthracis expression platform (1, 17, 30, 59), or by using a different animal model or B. anthracis strain for challenge studies, the potential or added value of these immunogens or of the remaining 76 immunogens could be determined more conclusively for the development of a new anthrax vaccine.
Supplementary Material
Acknowledgments
We thank Gila Friedman and Yossi Shlomovitch for excellent technical assistance.
Editor: D. L. Burns
Footnotes
Published ahead of print on 12 March 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aloni-Grinstein, R., O. Gat, Z. Altboum, B. Velan, S. Cohen, and A. Shafferman. 2005. Oral spore vaccine based on live attenuated nontoxinogenic Bacillus anthracis expressing recombinant mutant protective antigen. Infect. Immun. 73:4043-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altboum, Z., Y. Gozes, A. Barnea, A. Pass, M. White, and D. Kobiler. 2002. Postexposure prophylaxis against anthrax: evaluation of various treatment regimens in intranasally infected guinea pigs. Infect. Immun. 70:6231-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antelmann, H., H. Tjalsma, B. Voigt, S. Ohlmeier, S. Bron, S. J. M. Van Dijl, and M. Hecker. 2001. A proteomic view on genome-based signal peptide predictions. Genome Res. 11:1484-1502. [DOI] [PubMed] [Google Scholar]
- 4.Antelmann, H., R. C. Williams, M. Miethke, A. Wipat, D. Albrecht, C. R. Harwood, and M. Hecker. 2005. The extracellular and cytoplasmic proteomes of the non-virulent Bacillus anthracis strain UM23C1-2. Proteomics 5:3684-3695. [DOI] [PubMed] [Google Scholar]
- 5.Ariel, N., A. Zvi, H. Grosfeld, O. Gat, Y. Inbar, B. Velan, S. Cohen, and A. Shafferman. 2002. Search for potential vaccine candidate open reading frames in the Bacillus anthracis virulence plasmid pXO1: in silico and in vitro screening. Infect. Immun. 70:6817-6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariel, N., A. Zvi, K. Makarova, T. Chitlaru, E. Elhanany, B. Velan, S. Cohen, A. Friedlander, and A. Shafferman. 2003. Genome-based bioinformatic selection of chromosomal Bacillus anthracis putative vaccine candidates coupled with proteomic identification of surface-associated antigens. Infect. Immun. 71:4563-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballot, E., A. Bruneel, V. Labas, and C. Johanet. 2003. Identification of rat targets of anti-soluble liver antigen autoantibodies by serologic proteome analysis. Clin. Chem. 49:634-643. [DOI] [PubMed] [Google Scholar]
- 8.Banu, S., K. H. Ohtani, H. Yaguchi, T. Swe, S. T. Cole, H. Hayashi, and T. Shimizu. 2000. Identification of novel VirR/VirS-regulated genes in Clostridium perfringens. Mol. Microbiol. 35:854-864. [DOI] [PubMed] [Google Scholar]
- 9.Blendon, R. J., J. M. Benson, C. M. Des Roches, W. E. Pollard, C. Parvanta, and M. J. Herrmann. 2002. The impact of anthrax attacks on the American public. Med. Gen. Med. 4:1. [PubMed] [Google Scholar]
- 10.Bradley, K., J. Mogridge, M. Mourez, B. Collier, and J. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. [DOI] [PubMed] [Google Scholar]
- 11.Brossier, F., M. Levy, and M. Mock. 2002. Anthrax spores make an essential contribution to vaccine efficacy. Infect. Immun. 70:661-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchmeier, N. A., and F. Heffron. 1990. Induction of stress proteins upon infection of macrophages. Science 248:730-732. [DOI] [PubMed] [Google Scholar]
- 13.Cendrowski, S., W. MacArthur, and P. Hanna. 2004. Bacillus anthracis requires siderophore biosynthesis for growth in macrophages and mouse virulence. Mol. Microbiol. 551:407-417. [DOI] [PubMed] [Google Scholar]
- 14.Chakravarti, D., M. Fiske, L. Fletcher, and R. Zagursky. 2000. Application of genomics and proteomics for identification of bacterial gene products as potential vaccine candidates. Vaccine 19:601-612. [DOI] [PubMed] [Google Scholar]
- 15.Chitlaru, T., N. Ariel, A. Zvi, M. Lion, B. Velan, A. Shafferman, and E. Elhanany. 2004. Identification of chromosomally encoded membranal polypeptides of Bacillus anthracis by a proteomic approach—prevalence of proteins containing S-layer homology domains. Proteomics 4:677-691. [DOI] [PubMed] [Google Scholar]
- 16.Chitlaru, T., O. Gat, Y. Gozlan, N. Ariel, and A. Shafferman. 2006. Differential proteomic analysis of the Bacillus anthracis secretome: distinct plasmid and chromosome CO2-dependent cross-talk mechanisms modulate extracellular proteolytic activities. J. Bacteriol. 188:3551-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen, S., I. Mendelson, Z. Altboum, D. Kobiler, E. Elhanany, T. Bino, M. Leitner, I. Inbar, H. Rosenberg, Y. Gozes, R. Barak, M. Fisher, C. Kronman, B. Velan, and A. Shafferman. 2000. Attenuated nontoxinogenic and nonencapulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect. Immun. 68:4549-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes, G., B. de Astorza, V. J. Benedi, and A. Sebastián. 2002. Role of the htrA gene in Klebsiella pneumoniae virulence. Infect. Immun. 70:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davison, S., E. Couture-Tosi, T. Candela, M. Mock, and A. Fouet. 2005. Identification of the Bacillus anthracis γ phage receptor. J. Bacteriol. 187:6742-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DelVecchio, V. G., J. P. Connolly, T. G. Alefantis, A. Walz, M. A. Quan, G. Patra, J. M. Ashton, J. T. Whittington, R. D. Chafin, X. Liang, P. Grewal, A. S. Khan, and C. V. Mujer. 2006. Proteomic profiling and identification of immunodominant spore antigens of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Appl. Environ. Microbiol. 72:6355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fellows, P. F., M. K. Linscott, B. E. Ivins, M. L. M. Pitt, C. A. Rossi, P. H. Gibbs, and A. M. Friedlander. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 19:3241-3247. [DOI] [PubMed] [Google Scholar]
- 22.Fisher N., L. Shetron-Rama, A. Herring-Palmer, B. Heffernan, N. Bergman, and P. Hanna. 2006. The dltABCD operon of Bacillus anthracis Sterne is required for virulence and resistance to peptide, enzymatic, and cellular mediators of innate immunity. J. Bacteriol. 188:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folkesson, A., S. Eriksson, M. Andersson, J. T. Park, and S. Normark. 2005. Components of the peptidoglycan-recycling pathway modulate invasion and intracellular survival of Salmonella enterica serovar Typhimurium. Cell. Microbiol. 7:147-155. [DOI] [PubMed] [Google Scholar]
- 24.Fouet, A., S. Mesnage, E. Tosi-Couture, P. Gounon, and M. Mock. 1999. Bacillus anthracis surface: capsule and S-layer. J. Appl. Microbiol. 87:251-255. [DOI] [PubMed] [Google Scholar]
- 25.Friedlander, A., S. Welkos, and B. Ivins. 2002. Anthrax vaccines. Curr. Top. Microbiol. Immunol. 271:34-60. [DOI] [PubMed] [Google Scholar]
- 26.Gahan, C. G. M., J. O'Mahony, and C. Hill. 2001. Characterization of the GroESL operon in Listeria monocytogenes: utilization of two reporter systems (gfp and hly) for evaluating in vivo expression. Infect. Immun. 69:3924-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garmory, H. S., and R. W. Titball. 2004. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect. Immun. 72:6757-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gat, O., H. Grosfeld, N. Ariel, I. Inbar, G. Zaide, Y. Broder, A. Zvi, T. Chitlaru, Z. Altboum, D. Stein, S. Cohen, and A. Shafferman. 2006. Search for Bacillus anthracis potential vaccine candidates by a functional genomic-serologic screen. Infect. Immun. 74:3987-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gat, O., H. Grosfeld, and A. Shafferman. 2007. In vitro screen of bioinformatically selected Bacillus anthracis vaccine candidates by coupled transcription, translation and immunoprecipitation analysis. Methods Mol. Biol. 375:211-233. [DOI] [PubMed] [Google Scholar]
- 30.Gat, O., I. Inbar, R. Aloni-Grinstein, E. Zahavy, C. Kronman, I. Mendelson, S. Cohen, B. Velan, and A. Shafferman. 2003. Use of a promoter trap system in B. anthracis and B. subtilis for the development of recombinant protective antigen-based vaccines. Infect. Immun. 71:801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gat, O., I. Mendelson, T. Chitlaru, N. Ariel, Z. Altboum, H. Levy, S. Weiss, H. Grosfeld, S. Cohen, and A. Shafferman. 2005. The solute-binding component of a putative Mn(II) ABC transporter (MntA) is a novel Bacillus anthracis virulence determinant. Mol. Microbiol. 58:533-551. [DOI] [PubMed] [Google Scholar]
- 32.Gohar, M., N. Gilois, R. Graveline, C. Garreau, V. Sanchis, and D. Lereclus. 2005. A comparative study of B. cereus, B. thuringiensis and B. anthracis extracellular proteomes. Proteomics 5:3696-3711. [DOI] [PubMed] [Google Scholar]
- 33.Grandi, G. 2003. Rational antibacterial vaccine design through genomic technologies. Int. J. Parasitol. 33:615-620. [DOI] [PubMed] [Google Scholar]
- 34.Grosfeld, H., S. Cohen, T. Bino, Y. Flashner, R. Ber, E. Mamroud, C. Kronman, A. Shafferman, and B. Velan. 2003. Effective protective immunity to Yersinia pestis infection conferred by DNA vaccine coding for derivatives of the F1 capsular antigen. Infect. Immun. 71:374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas, G., G. Karaali, K. Ebermayer, W. G. Metzger, S. Lamer, U. Zimny-Arndt, S. Diescher, U. B. Goebel, K. Vogt, A. B. Roznowski, B. Wiedenmann, T. F. Meyer, T. Aebischer, and P. R. Jungblut. 2002. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics 2:313-324. [DOI] [PubMed] [Google Scholar]
- 36.Havlasova, J., L. Hernychova, P. Halada, V. Pellantova, J. Krejsek, J. Stulik, A. Macela, P. Jungblut, P. Larsson, and M. Forsman. 2002. Mapping of immunoreactive antigens of Francisella tularensis live vaccine strain. Proteomics 2:857-867. [DOI] [PubMed] [Google Scholar]
- 37.Heffernan, B., B. Thomason, A. Herring-Palmer, L. Shaughnessy, R. McDonald, N. Fisher, G. Huffnagle, and P. Hanna. 2006. Bacillus anthracis phospholipases C facilitate macrophage-associated growth and contribute to virulence in a murine model of inhalation anthrax. Infect. Immun. 74:3756-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henriques, A. O., B. W. Beal, K. Ronald, and C. P. Moran, Jr. 1995. Characterization of cotJ, a sigma E-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J. Bacteriol. 177:3394-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang, C. M., K. W. Foster, T. S. DeSilva, K. R. Van Kampen, C. A. Elmets, and D. C. Tang. 2004. Identification of Bacillus anthracis proteins associated with germination and early outgrowth by proteomic profiling of anthrax spores. Proteomics 4:2653-2661. [DOI] [PubMed] [Google Scholar]
- 40.Hyyrylainen, H. L., A. Bolhuis, E. Darmon, L. Muukonen, P. Koski, M. Vitikainen, M. Sarvas, Z. Pragai, S. Bron, J. M. Van Dijl, and V. P. Kontinen. 2001. A novel two-component regulatory system in Bacillus subtilis for the survival of severe secretion stress. Mol. Microbiol. 41:1159-1172. [DOI] [PubMed] [Google Scholar]
- 41.Ibrahim, Y. M., A. R. Kerr, J. McCluskey, and T. J. Mitchell. 2004. Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect. Immun. 72:3584-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivins, B. E., and S. L. Welkos. 1988. Recent advances in the development of an improved, human anthrax vaccine. Eur. J. Epidemiol. 4:12-19. [DOI] [PubMed] [Google Scholar]
- 43.Klade, C. S., T. Voss, E. Krystek, H. Ahorn, K. Zaltoukal, K. Pummer, and G. Adolf. 2001. Identification of tumor antigens in renal cell carcinoma by serological proteome analysis. Proteomics 2001:890-898. [DOI] [PubMed] [Google Scholar]
- 44.Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13:1093-1100. [DOI] [PubMed] [Google Scholar]
- 45.Kobiler, D., Y. Gozes, H. Rosenberg, S. Reuveny, and Z. Altboum. 2002. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect. Immun. 70:544-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kornilovs'ka, I., I. Nilsson, M. Utt, Å. Ljungh, and T. Wadström. 2002. Immunogenic proteins of Helicobacter pullorum, Helicobacter bilis and Helicobacter hepaticus identified by two-dimensional gel electrophoresis and immunoblotting. Proteomics 2:775-783. [DOI] [PubMed] [Google Scholar]
- 47.Kudva, I. T., R. W. Griffin, J. M. Garren, S. B. Calderwood, and M. John. 2005. Identification of a protein subset of the anthrax spore immunome in humans immunized with the anthrax vaccine adsorbed preparation. Infect. Immun. 73:5685-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacy, T. M., and R. J. Collier. 2002. Structure and function of anthrax toxin. Curr. Top. Microbiol. Immunol. 271:62-85. [DOI] [PubMed] [Google Scholar]
- 49.Lamonica, J. M., M. Wagner, M. Eschenbrenner, L. E. Williams, T. L. Miller, G. Patra, and V. G. DelVecchio. 2005. Comparative secretome analyses of three Bacillus anthracis strains with variant plasmid contents. Infect. Immunol. 73:3646-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee, V. T., and O. Schneewind. 2001. Protein secretion and the pathogenesis of bacterial infections. Genes Dev. 15:1725-1752. [DOI] [PubMed] [Google Scholar]
- 51.Leppla, S. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leppla, S. 1995. Anthrax toxins, p. 543-572. In J. Moss, B. Iglewski, M. Vaughan, and A. T. Tu (ed.), Bacterial toxins and virulence factors in disease. Marcel Dekker, New York, NY.
- 53.Leppla, S. 1999. The bifactorial B. anthracis lethal and oedema toxins, p. 243-263. In J. E. Alouf and J. H. Freer (ed.), Comprehensive sourcebook of bacterial protein toxins. Academic Press, London, United Kingdom.
- 54.Leppla, S., J. Robbins, R. Schneerson, and J. Shiloach. 2002. Development of an improved vaccine for anthrax. J. Clin. Investig. 110:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li, S.-R., N. Dorrell, P. H. Everest, G. Dougan, and B. W. Wren. 1996. Construction and characterization of a Yersinia enterocolitica O:8 high-temperature requirement (htrA) isogenic mutant. Infect. Immun. 64:2088-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu, H., N. H. Bergman, B. Thomason, S. Shallom, A. Hazen, J. Crossno, D. Rasko, J. Ravel, T. D. Read, S. N. Petterson, J. Yates III, and P. C. Hanna. 2004. Formation and composition of the Bacillus anthracis endospore. J. Bacteriol. 186:164-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loosmore, S. M., Y.-P. Yang, R. Oomen, J. M. Shortreed, D. C. Coleman, and M. H. Klein. 1998. The Haemophilus influenzae HtrA protein is a protective antigen. Infect. Immun. 66:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makino, S., I. Uchida, N. Terakado, C. Sasakawa, and M. Yoshikawa. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendelson, I., O. Gat, R. Aloni-Grinstein, Z. Altboum, I. Inbar, C. Kronman, E. Bar-Haim, S. Cohen, B. Velan, and A. Shafferman. 2005. Efficacious, nontoxigenic Bacillus anthracis spore vaccines based on strains expressing mutant variants of lethal toxin components. Vaccine 23:5688-5697. [DOI] [PubMed] [Google Scholar]
- 60.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 61.Okinaka, R., K. Cloud, O. Hampton, A. Hoffmaster, K. Hill, P. Keim, T. Koehler, G. Lamke, S. Kumano, D. Manter, Y. Martinez, D. Ricke, R. Svensson, and P. Jackson. 1999. Sequence, assembly and analysis of pXO1 and pXO2. J. Appl. Microbiol. 87:261-262. [DOI] [PubMed] [Google Scholar]
- 62.Olczak, A., R. W. Seyler, J. Olson, and R. Maier. 2003. Association of Helicobacter pylori antioxidant activities with host colonization proficiency. Infect. Immun. 71:580-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Passalacqua, K. D., N. H. Bergman, A. Herring-Palmer, and P. Hanna. 2006. The superoxide dismutase of Bacillus anthracis does not cooperatively protect against endogenous superoxide stress. J. Bacteriol. 188:3837-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pessolani, M. C. V., and P. Brennan. 1996. Molecular definition and identification of new proteins of Mycobacterium leprae. Infect. Immun. 64:5425-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pezard, C., M. Weber, J.-C. Sirard, P. Berche, and M. Mock. 1995. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect. Immun. 63:1369-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Aricò, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 67.Price, B. M., A. L. Liner, S. Park, S. H. Leppla, A. Mateczun, and D. R. Galloway. 2001. Protection against lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect. Immun. 69:4509-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rankin, S., Z. Li, and R. Isberg. 2002. Macrophage-induced genes of Legionella pneumophila: protection from reactive intermediates and solute imbalance during intracellular growth. Infect. Immun. 70:3637-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Read, T. D., S. N. Peterson, N. Tourasse, L. W. Baillie, I. T. Paulsen, K. E. Nelson, H. Tettelin, D. E. Fouts, J. A. Eisen, S. R. Gill, E. K. Holtzapple, O. A. Okstad, E. Helgason, J. Rilstone, M. Wu, J. F. Kolonay, M. J. Beanan, R. J. Dodson, L. M. Brinkac, M. Gwinn, R. T. DeBoy, R. Madpu, S. C. Daugherty, A. S. Durkin, D. H. Haft, W. C. Nelson, J. D. Peterson, M. Pop, H. M. Khouri, D. Radune, J. L. Benton, Y. Mahamoud, L. Jiang, I. R. Hance, J. F. Weidman, K. J. Berry, R. D. Plaut, A. M. Wolf, K. L. Watkins, W. C. Nierman, A. Hazen, R. Cline, C. Redmond, J. E. Thwaite, O. White, S. L. Salzberg, B. Thomason, A. M. Friedlander, T. M. Koehler, P. C. Hanna, A. B. Kolsto, and C. M. Fraser. 2003. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 423:81-86. [DOI] [PubMed] [Google Scholar]
- 70.Ross, C., and T. Koehler. 2006. plcR papR-Independent expression of anthrolysin O by Bacillus anthracis. J. Bacteriol. 188:7823-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanchez-Campillo, M., L. Bini, M. Comanducci, R. Raggiaschi, B. Marzocchi, V. Pallini, and G. Ratti. 1999. Identification of immunoreactive proteins of Chlamydia trachomatis by Western blot analysis of a two-dimensional electrophoresis map with patient sera. Electrophoresis 20:2269-2279. [DOI] [PubMed] [Google Scholar]
- 72.Schneerson, R., J. Kubler-Kielb, T.-Y. Liu, Z.-D. Dai, S. Leppla, A. Yergey, P. Backlund, J. Shiloach, F. Majadly, and J. Robbins. 2003. Poly-d-glutamic acid protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc. Natl. Acad. Sci. USA. 100:8945-8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh, Y., K. Klimpel, S. Goel, P. Swain, and S. Leppla. 1999. Oligomerization of anthrax toxin protective antigen and binding of lethal factor during endocytic uptake into mammalian cells. Infect. Immun. 67:1853-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith, I. 2003. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 16:463-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutcliffe, I., and D. Harrington. 2002. Pattern searches for the identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiology 148:2065-2077. [DOI] [PubMed] [Google Scholar]
- 76.Taylor, P. D., C. J. Inchley, and M. Gallagher. 1998. Salmonella typhimurium AhpC polypeptide is not essential for virulence in BALB/c mice but is recognized as an antigen during infection. Infect. Immun. 66:3208-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tjalsma, H., A. Bolhuis, D. H. Jongbloed, S. Bron, and J. M. Van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tjalsma, H., H. Antelman, J. D. H. Jongbloed, P. G. Braun, E. Darmon, R. Dorenbos, J.-Y. F. Dubois, H. Westers, G. Zanen, W. J. Quax, O. P. Kuipers, S. Bron, M. Hecker, and J. M. Van Dijl. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68:207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Todd, S. J., A. J. G. Moir, M. J. Johnson, and A. Moir. 2003. Genes of Bacillus cereus and Bacillus anthracis encoding proteins of the exosporium. J. Bacteriol. 185:3373-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Twine, S. M., M. D. Petit, H. Shen, N. C. S. Mykytczuk, J. F. Kelly, and J. W. Conlan. 2006. Immunoproteomic analysis of the murine antibody response to successful and failed immunization with live anti-Francisella vaccines. Biochem. Biophys. Res. Commun. 345:1621-1633. [DOI] [PubMed] [Google Scholar]
- 81.Van Dijl, J. M., P. G. Braun, C. Robinson, W. J. Quax, H. Antelman, M. Hecker, J. Muller, H. Tjalsma, S. Bron, and J. D. H. Jongbloed. 2002. Functional genomic analysis of the Bacillus subtilis Tat pathway for protein secretion. J. Biotechnol. 98:243-254. [DOI] [PubMed] [Google Scholar]
- 82.Vytvytska, O., E. Nagy, M. Blüggel, H. Meyer, R. Kurzbauer, L. Huber, and C. S. Klade. 2002. Identification of vaccine candidate antigens of Staphylococcus aureus by serological proteome analysis. Proteomics 2:580-590. [DOI] [PubMed] [Google Scholar]
- 83.Whiting, G. C., S. Rijpkema, T. Adams, and M. Corbel. 2004. Characterisation of adsorbed anthrax vaccine by two-dimensional gel electrophoresis. Vaccine 22:4245-4251. [DOI] [PubMed] [Google Scholar]
- 84.Wright, G., M. Puzzis, and B. Neely. 1962. Studies on immunity in anthrax. J. Bacteriol. 83:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Young, G., and V. Miller. 1997. Identification of novel chromosomal loci affecting Yersinia enterocolitica pathogenesis. Mol. Microbiol. 25:319-328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.